COVID-19, post-acute COVID-19 syndrome (PACS, "long COVID") and post-COVID-19 vaccination syndrome (PCVS, "post-COVIDvac-syndrome"): Similarities and differences
- PMID: 37192595
- PMCID: PMC10154064
- DOI: 10.1016/j.prp.2023.154497
COVID-19, post-acute COVID-19 syndrome (PACS, "long COVID") and post-COVID-19 vaccination syndrome (PCVS, "post-COVIDvac-syndrome"): Similarities and differences
Abstract
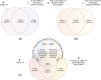
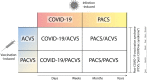
Worldwide there have been over 760 million confirmed coronavirus disease 2019 (COVID-19) cases, and over 13 billion COVID-19 vaccine doses have been administered as of April 2023, according to the World Health Organization. An infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can lead to an acute disease, i.e. COVID-19, but also to a post-acute COVID-19 syndrome (PACS, "long COVID"). Currently, the side effects of COVID-19 vaccines are increasingly being noted and studied. Here, we summarise the currently available indications and discuss our conclusions that (i) these side effects have specific similarities and differences to acute COVID-19 and PACS, that (ii) a new term should be used to refer to these side effects (post-COVID-19 vaccination syndrome, PCVS, colloquially "post-COVIDvac-syndrome"), and that (iii) there is a need to distinguish between acute COVID-19 vaccination syndrome (ACVS) and post-acute COVID-19 vaccination syndrome (PACVS) - in analogy to acute COVID-19 and PACS ("long COVID"). Moreover, we address mixed forms of disease caused by natural SARS-CoV-2 infection and COVID-19 vaccination. We explain why it is important for medical diagnosis, care and research to use the new terms (PCVS, ACVS and PACVS) in order to avoid confusion and misinterpretation of the underlying causes of disease and to enable optimal medical therapy. We do not recommend to use the term "Post-Vac-Syndrome" as it is imprecise. The article also serves to address the current problem of "medical gaslighting" in relation to PACS and PCVS by raising awareness among the medical professionals and supplying appropriate terminology for disease.
Keywords: ACVS; Acute COVID-19 vaccination syndrome; COVID-19; Long COVID; PACS; PACVS; PCVS; Post-COVID-19 vaccination syndrome; Post-acute COVID-19 syndrome; Post-acute COVID-19 vaccination syndrome; SARS-CoV-2.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- WHO Pneumonia of unknown cause – China. 2020.
-
- Gao Y.D., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

