USP2 promotes experimental colitis and bacterial infections by inhibiting the proliferation of myeloid cells and remodeling the extracellular matrix network
- PMID: 37192862
- PMCID: PMC10120320
- DOI: 10.1016/j.cellin.2022.100047
USP2 promotes experimental colitis and bacterial infections by inhibiting the proliferation of myeloid cells and remodeling the extracellular matrix network
Erratum in
-
Corrigendum to previous published articles.Cell Insight. 2025 Jan 11;4(2):100225. doi: 10.1016/j.cellin.2024.100225. eCollection 2025 Apr. Cell Insight. 2025. PMID: 39881711 Free PMC article.
Abstract
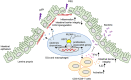
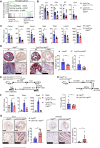
Inflammatory bowel disease (IBD) is closely associated with dysregulation of genetic factors and microbial environment. Here, we report a susceptible role of ubiquitin-specific protease 2 (USP2) in experimental colitis and bacterial infections. USP2 is upregulated in the inflamed mucosa of IBD patients and in the colon of mice treated with dextran sulfate sodium salt (DSS). Knockout or pharmacologic inhibition of USP2 promotes the proliferation of myeloid cells to activate IL-22 and IFNγ production of T cells. In addition, knockout of USP2 in myeloid cells inhibits the production of pro-inflammatory cytokines to relieve the dysregulation of extracellular matrix (ECM) network and promote the gut epithelial integrity after DSS treatment. Consistently, Lyz2-Cre;Usp2fl/fl mice exhibit hyper-resistance to DSS-induced colitis and Citrobacter rodentium infections compared to Usp2fl/fl mice. These findings highlight an indispensable role of USP2 in myeloid cells to modulate T cell activation and epithelial ECM network and repair, indicating USP2 as a potential target for therapeutic intervention of IBD and bacterial infections in the gastrointestinal system.
Keywords: Bacterial infection; Cell proliferation; Colitis; Myeloid cells; USP2.
© 2022 The Authors.
Figures






References
-
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Ananthakrishnan A.N., Bernstein C.N., Iliopoulos D., Macpherson A., Neurath M.F., Ali R.A.R., Vavricka S.R., Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018;15:39–49. - PubMed
-
- Andres V., Pello O.M., Silvestre-Roig C. Macrophage proliferation and apoptosis in atherosclerosis. Curr. Opin. Lipidol. 2012;23:429–438. - PubMed
-
- Bassler K., Schulte-Schrepping J., Warnat-Herresthal S., Aschenbrenner A.C., Schultze J.L. The myeloid cell compartment-cell by cell. Annu. Rev. Immunol. 2019;37:269–293. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

