Intertwined regulation between RNA m6A modification and cancer metabolism
- PMID: 37192910
- PMCID: PMC10120304
- DOI: 10.1016/j.cellin.2022.100075
Intertwined regulation between RNA m6A modification and cancer metabolism
Erratum in
-
Erratum regarding missing declaration of interests in previously published articles.Cell Insight. 2024 Jan 30;3(1):100148. doi: 10.1016/j.cellin.2024.100148. eCollection 2024 Feb. Cell Insight. 2024. PMID: 38323319 Free PMC article.
Abstract
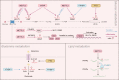
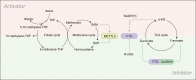
RNA N6-methyladenosine (m6A) has been identified as the most common, abundant and conserved internal modification in RNA transcripts, especially within eukaryotic messenger RNAs (mRNAs). Accumulating evidence demonstrates that RNA m6A modification exploits a wide range of regulatory mechanisms to control gene expression in pathophysiological processes including cancer. Metabolic reprogramming has been widely recognized as a hallmark of cancer. Cancer cells obtain metabolic adaptation through a variety of endogenous and exogenous signaling pathways to promote cell growth and survival in the microenvironment with limited nutrient supply. Recent emerging evidence reveals reciprocal regulation between the m6A modification and disordered metabolic events in cancer cells, adding more complexity in the cellular network of metabolic rewiring. In this review, we summarize the most recent advances of how RNA methylation affects tumor metabolism and the feedback regulation of m6A modification by metabolic intermediates. We aim to highlight the important connection between RNA m6A modification and cancer metabolism, and expect that studise of RNA m6A and metabolic reprogramming will lead to greater understanding of cancer pathology.
© 2022 The Authors.
Figures
References
-
- Aik W., Demetriades M., Hamdan M.K.K., Bagg E.A.L., Yeoh K.K., Lejeune C., Zhang Z., McDonough M.A., Schofield C.J. Structural basis for inhibition of the fat mass and obesity associated protein (FTO) Journal of Medicinal Chemistry. 2013;56:3680–3688. - PubMed
-
- Bhutia Y.D., Babu E., Ramachandran S., Ganapathy V. Amino acid transporters in cancer and their relevance to "glutamine addiction": Novel targets for the design of a new class of anticancer drugs. Cancer Research. 2015;75:1782–1788. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

