Regulation and function of the cGAS-MITA/STING axis in health and disease
- PMID: 37192983
- PMCID: PMC10120319
- DOI: 10.1016/j.cellin.2021.100001
Regulation and function of the cGAS-MITA/STING axis in health and disease
Erratum in
-
Corrigendum to previous published articles.Cell Insight. 2025 Jan 11;4(2):100225. doi: 10.1016/j.cellin.2024.100225. eCollection 2025 Apr. Cell Insight. 2025. PMID: 39881711 Free PMC article.
Abstract
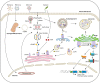
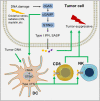
The innate immune systems detect pathogens via pattern-recognition receptors including nucleic acid sensors and non-nucleic acid sensors. Cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS, also known as MB21D1) is a cytosolic DNA sensor that recognizes double-stranded DNA (dsDNA) and catalyzes the synthesis of 2',3'-cGAMP. Subsequently, 2',3'-cGAMP binds to the adaptor protein mediator of IRF3 activation (MITA, also known as STING, MPYS, ERIS, and TMEM173) to activate downstream signaling cascades. The cGAS-MITA/STING signaling critically mediates immune responses against DNA viruses, retroviruses, bacteria, and protozoan parasites. In addition, recent discoveries have extended our understanding of the roles of the cGAS-MITA/STING pathway in autoimmune diseases and cancers. Here, we summarize the identification and activation of cGAS and MITA/STING, present the updated functions and regulatory mechanisms of cGAS-MITA/STING signaling and provide a comprehensive understanding of the cGAS-MITA/STING axis in autoimmune diseases and cancers.
Keywords: Autoimmune diseases; Cancer; Innate immunity; MITA/STING; cGAS.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Ahn J., Barber G.N. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Current Opinion in Immunology. 2014;31:121–126. - PubMed
-
- Andreeva L., Hiller B., Kostrewa D., Lassig C., de Oliveira Mann C.C., Jan Drexler D., Maiser A., Gaidt M., Leonhardt H., Hornung V., et al. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature. 2017;549:394–398. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

