A global federated real-world data and analytics platform for research
- PMID: 37193038
- PMCID: PMC10182857
- DOI: 10.1093/jamiaopen/ooad035
A global federated real-world data and analytics platform for research
Abstract
Objective: This article describes a scalable, performant, sustainable global network of electronic health record data for biomedical and clinical research.
Materials and methods: TriNetX has created a technology platform characterized by a conservative security and governance model that facilitates collaboration and cooperation between industry participants, such as pharmaceutical companies and contract research organizations, and academic and community-based healthcare organizations (HCOs). HCOs participate on the network in return for access to a suite of analytics capabilities, large networks of de-identified data, and more sponsored trial opportunities. Industry participants provide the financial resources to support, expand, and improve the technology platform in return for access to network data, which provides increased efficiencies in clinical trial design and deployment.
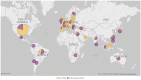
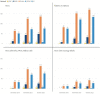
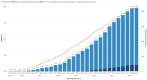
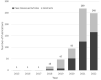
Results: TriNetX is a growing global network, expanding from 55 HCOs and 7 countries in 2017 to over 220 HCOs and 30 countries in 2022. Over 19 000 sponsored clinical trial opportunities have been initiated through the TriNetX network. There have been over 350 peer-reviewed scientific publications based on the network's data.
Conclusions: The continued growth of the TriNetX network and its yield of clinical trial collaborations and published studies indicates that this academic-industry structure is a safe, proven, sustainable path for building and maintaining research-centric data networks.
Keywords: clinical trial protocols; data management; data warehouse; electronic health records; real-world data.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
MBP, ZJD, JPW-J, CNT, JE, and BC are employees of TriNetX, LLC. JWL and DP-R have a Consulting Role with TriNetX, LLC.
Figures

References
-
- Califf RM. The Patient-Centered Outcomes Research Network: a national infrastructure for comparative effectiveness research. N C Med J 2014; 75 (3): 204–10. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
