Current advances of CRISPR-Cas technology in cell therapy
- PMID: 37193354
- PMCID: PMC10120314
- DOI: 10.1016/j.cellin.2022.100067
Current advances of CRISPR-Cas technology in cell therapy
Erratum in
-
Corrigendum to previous published articles.Cell Insight. 2025 Jan 11;4(2):100225. doi: 10.1016/j.cellin.2024.100225. eCollection 2025 Apr. Cell Insight. 2025. PMID: 39881711 Free PMC article.
Abstract
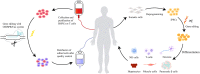
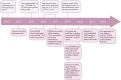
CRISPR-Cas is a versatile genome editing technology that has been broadly applied in both basic research and translation medicine. Ever since its discovery, the bacterial derived endonucleases have been engineered to a collection of robust genome-editing tools for introducing frameshift mutations or base conversions at site-specific loci. Since the initiation of first-in-human trial in 2016, CRISPR-Cas has been tested in 57 cell therapy trials, 38 of which focusing on engineered CAR-T cells and TCR-T cells for cancer malignancies, 15 trials of engineered hematopoietic stem cells treating hemoglobinopathies, leukemia and AIDS, and 4 trials of engineered iPSCs for diabetes and cancer. Here, we aim to review the recent breakthroughs of CRISPR technology and highlight their applications in cell therapy.
Keywords: CRISPR; Cas9; Cell therapy; Genome editing; HSPC; T cell; iPSC.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Albitar M., Care A., Peschle C., Liebhaber S.A. Developmental switching of messenger RNA expression from the human alpha-globin cluster: fetal/adult pattern of theta-globin gene expression. Blood. 1992;80:1586–1591. - PubMed
-
- Allers K., Hutter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E., Schneider T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
-
- Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–1208. - PubMed
-
- Anzalone A.V., Koblan L.W., Liu D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020;38:824–844. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

