A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides
- PMID: 37193692
- PMCID: PMC10188516
- DOI: 10.1038/s41467-023-38215-z
A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides
Erratum in
-
Publisher Correction: A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides.Nat Commun. 2023 Jun 23;14(1):3747. doi: 10.1038/s41467-023-39001-7. Nat Commun. 2023. PMID: 37353544 Free PMC article. No abstract available.
Abstract
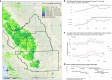
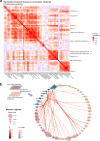
Parkinson's disease (PD) is a complex neurodegenerative disease with etiology rooted in genetic vulnerability and environmental factors. Here we combine quantitative epidemiologic study of pesticide exposures and PD with toxicity screening in dopaminergic neurons derived from PD patient induced pluripotent stem cells (iPSCs) to identify Parkinson's-relevant pesticides. Agricultural records enable investigation of 288 specific pesticides and PD risk in a comprehensive, pesticide-wide association study. We associate long-term exposure to 53 pesticides with PD and identify co-exposure profiles. We then employ a live-cell imaging screening paradigm exposing dopaminergic neurons to 39 PD-associated pesticides. We find that 10 pesticides are directly toxic to these neurons. Further, we analyze pesticides typically used in combinations in cotton farming, demonstrating that co-exposures result in greater toxicity than any single pesticide. We find trifluralin is a driver of toxicity to dopaminergic neurons and leads to mitochondrial dysfunction. Our paradigm may prove useful to mechanistically dissect pesticide exposures implicated in PD risk and guide agricultural policy.
© 2023. The Author(s).
Conflict of interest statement
L.L.R. is a founder of and a member of the Scientific Advisory Board of Vesalius Therapeutics, a private biotechnology company, and an owner of stock options. He is a member of the Scientific Advisory Board of Yumanity Therapeutics and a shareholder. Both companies study Parkinson’s disease. B.R., M.C., and R.C.K. have been retained as expert consultants for plaintiffs in a lawsuit on the role of paraquat in Parkinson’s disease causation. V.K. is a co-founder of and senior advisor to DaCapo Brainscience and Yumanity Therapeutics, companies focused on central nervous system diseases. The remaining authors declare no competing interests.
Figures




References
-
- Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. - PubMed
-
- Goldman SM, Musgrove RE, Jewell SA, Di Monte DA. Chapter Three - Pesticides and Parkinson’s Disease: Current Experimental and Epidemiological Evidence. In: Advances in Neurotoxicology (eds Aschner M, Costa LG). Academic Press (2017).
-
- California Department of Pesticide Regulation. Actively Registered Active Ingredients (AI) by Common Name.).

