Protection against SARS-CoV-2 Omicron BA.4/5 variant following booster vaccination or breakthrough infection in the UK
- PMID: 37193713
- PMCID: PMC10187514
- DOI: 10.1038/s41467-023-38275-1
Protection against SARS-CoV-2 Omicron BA.4/5 variant following booster vaccination or breakthrough infection in the UK
Abstract
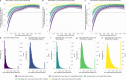
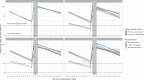
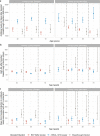
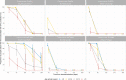
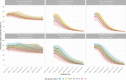
Following primary SARS-CoV-2 vaccination, whether boosters or breakthrough infections provide greater protection against SARS-CoV-2 infection is incompletely understood. Here we investigated SARS-CoV-2 antibody correlates of protection against new Omicron BA.4/5 (re-)infections and anti-spike IgG antibody trajectories after a third/booster vaccination or breakthrough infection following second vaccination in 154,149 adults ≥18 y from the United Kingdom general population. Higher antibody levels were associated with increased protection against Omicron BA.4/5 infection and breakthrough infections were associated with higher levels of protection at any given antibody level than boosters. Breakthrough infections generated similar antibody levels to boosters, and the subsequent antibody declines were slightly slower than after boosters. Together our findings show breakthrough infection provides longer-lasting protection against further infections than booster vaccinations. Our findings, considered alongside the risks of severe infection and long-term consequences of infection, have important implications for vaccine policy.
© 2023. The Author(s).
Conflict of interest statement
D.W.E. declares lecture fees from Gilead, outside the submitted work. P.C.M. receives GSK funding to support a PhD fellowship in her team. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

