FGF21 modulates hippocampal cold-shock proteins and CA2-subregion proteins in neonatal mice with hypoxia-ischemia
- PMID: 37193753
- PMCID: PMC10690493
- DOI: 10.1038/s41390-023-02652-9
FGF21 modulates hippocampal cold-shock proteins and CA2-subregion proteins in neonatal mice with hypoxia-ischemia
Abstract
Background: Fibroblast growth factor 21 (FGF21) is a neuroprotectant with cognitive enhancing effects but with poorly characterized mechanism(s) of action, particularly in females. Prior studies suggest that FGF21 may regulate cold-shock proteins (CSPs) and CA2-marker proteins in the hippocampus but empirical evidence is lacking.
Methods: We assessed in normothermic postnatal day (PND) 10 female mice, if hypoxic-ischemic (HI) brain injury (25 min 8% O2/92% N2) altered endogenous levels of FGF21 in serum or in the hippocampus, or its receptor β-klotho. We also tested if systemic administration of FGF21 (1.5 mg/kg) modulated hippocampal CSPs or CA2 proteins. Finally, we measured if FGF21 therapy altered markers of acute hippocampal injury.
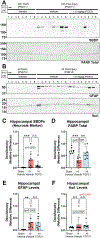
Results: HI increased endogenous serum FGF21 (24 h), hippocampal tissue FGF21 (4d), and decreased hippocampal β-klotho levels (4d). Exogenous FGF21 therapy modulated hippocampal CSP levels, and dynamically altered hippocampal CA2 marker expression (24 h and 4d). Finally, FGF21 ameliorated neuronal damage markers at 24 h but did not affect GFAP (astrogliosis) or Iba1 (microgliosis) levels at 4d.
Conclusions: FGF21 therapy modulates CSP and CA2 protein levels in the injured hippocampus. These proteins serve different biological functions, but our findings suggest that FGF21 administration modulates them in a homeostatic manner after HI.
Impact: Hypoxic-ischemic (HI) injury in female post-natal day (PND) 10 mice decreases hippocampal RNA binding motif 3 (RBM3) levels in the normothermic newborn brain. HI injury in normothermic newborn female mice alters serum and hippocampal fibroblast growth factor 21 (FGF21) levels 24 h post-injury. HI injury in normothermic newborn female mice alters hippocampal levels of N-terminal EF-hand calcium binding protein 2 (NECAB2) in a time-dependent manner. Exogenous FGF21 therapy ameliorates the HI-mediated loss of hippocampal cold-induced RNA-binding protein (CIRBP). Exogenous FGF21 therapy modulates hippocampal levels of CA2-marker proteins after HI.
© 2023. The Author(s), under exclusive licence to the International Pediatric Research Foundation, Inc.
Figures





References
-
- Lawn JE et al. Every Newborn: Progress, Priorities, and Potential Beyond Survival. Lancet 384, 189–205 (2014). - PubMed
-
- Acun C et al. Trends of Neonatal Hypoxic-Ischemic Encephalopathy Prevalence and Associated Risk Factors in the United States, 2010 to 2018. Am J Obstet Gynecol (2022). - PubMed
-
- Sandoval Karamian AG et al. Neonatal Encephalopathy: Etiologies Other Than Hypoxic-Ischemic Encephalopathy. Semin Fetal Neonatal Med 26, 101272 (2021). - PubMed
-
- Schiering IA et al. Correlation between Clinical and Histologic Findings in the Human Neonatal Hippocampus after Perinatal Asphyxia. J Neuropathol Exp Neurol 73, 324–334 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

