Erosion-inhibiting potential of the stannous fluoride-enriched CPP-ACP complex in vitro
- PMID: 37193788
- PMCID: PMC10188480
- DOI: 10.1038/s41598-023-34884-4
Erosion-inhibiting potential of the stannous fluoride-enriched CPP-ACP complex in vitro
Abstract
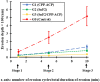
Currently available anti-erosive agents only provide partial protection, emphasizing the need to enhance their performance. By characterizing erosive enamel wear at the nanoscale, the aim of this in vitro study was to assess the anti-erosive effects of SnF2 and CPP-ACP both individually and synergistically. Erosion depths were assessed longitudinally on 40 polished human enamel specimens after 1, 5, and 10 erosion cycles. Each cycle comprised one-min erosion in citric acid (pH 3.0) and one-min treatment in whole saliva (control group) or a slurry of one of the three anti-erosive pastes (10% CPP-ACP; 0.45% SnF2 (1100 ppm F); or SnF2/CPP-ACP (10% CPP-ACP + 0.45% SnF2)) (n = 10 per group). Scratch depths were assessed longitudinally in separate experiments using a similar protocol after 1, 5, and 10 cycles. Compared with the control groups, all slurries reduced erosion depths after 1 cycle (p ≤ 0.004) and scratch depths after 5 cycles (p ≤ 0.012). The order of anti-erosive potential was SnF2/CPP-ACP > SnF2 > CPP-ACP > control for erosion depth analysis, and SnF2/CPP-ACP > (SnF2 = CPP-ACP) > control for scratch depth analysis. These data provide 'proof of concept' evidence that SnF2/CPP-ACP has superior anti-erosive potential compared to SnF2 or CPP-ACP alone.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

