In-ovo echocardiography for application in cardiovascular research
- PMID: 37193927
- PMCID: PMC10188421
- DOI: 10.1007/s00395-023-00989-0
In-ovo echocardiography for application in cardiovascular research
Abstract
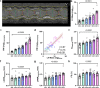
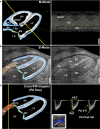
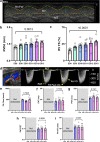
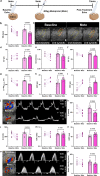
Preclinical cardiovascular research relies heavily on non-invasive in-vivo echocardiography in mice and rats to assess cardiac function and morphology, since the complex interaction of heart, circulation, and peripheral organs are challenging to mimic ex-vivo. While n-numbers of annually used laboratory animals worldwide approach 200 million, increasing efforts are made by basic scientists aiming to reduce animal numbers in cardiovascular research according to the 3R's principle. The chicken egg is well-established as a physiological correlate and model for angiogenesis research but has barely been used to assess cardiac (patho-) physiology. Here, we tested whether the established in-ovo system of incubated chicken eggs interfaced with commercially available small animal echocardiography would be a suitable alternative test system in experimental cardiology. To this end, we defined a workflow to assess cardiac function in 8-13-day-old chicken embryos using a commercially available high resolution ultrasound system for small animals (Vevo 3100, Fujifilm Visualsonics Inc.) equipped with a high frequency probe (MX700; centre transmit: 50 MHz). We provide detailed standard operating procedures for sample preparation, image acquisition, data analysis, reference values for left and right ventricular function and dimensions, and inter-observer variabilities. Finally, we challenged incubated chicken eggs with two interventions well-known to affect cardiac physiology-metoprolol treatment and hypoxic exposure-to demonstrate the sensitivity of in-ovo echocardiography. In conclusion, in-ovo echocardiography is a feasible alternative tool for basic cardiovascular research, which can easily be implemented into the small animal research environment using existing infrastructure to replace mice and rat experiments, and thus, reduce use of laboratory animals according to the 3R principle.
Keywords: 3R; Alternative methods; Chicken embryo; Echocardiography; Preclinical research.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–276. doi: 10.1164/rccm.201004-0601OC. - DOI - PubMed
-
- Beyhoff N, Brix S, Betz IR, Klopfleisch R, Foryst-Ludwig A, Krannich A, Stawowy P, Knebel F, Grune J, Kintscher U (2017) Application of speckle-tracking echocardiography in an experimental model of isolated subendocardial damage. J Am Soc Echocardiogr 30:1239–1250 e1232 10.1016/j.echo.2017.08.006 - PubMed
-
- Bhattacharya PT, Troutman GS, Mao F, Fox AL, Tanna MS, Zamani P, Grandin EW, Menachem JN, Birati EY, Chirinos JA, Mazimba S, Smith KA, Kawut SM, Forfia PR, Vaidya A, Mazurek JA. Right ventricular outflow tract velocity time integral-to-pulmonary artery systolic pressure ratio: a non-invasive metric of pulmonary arterial compliance differs across the spectrum of pulmonary hypertension. Pulm Circ. 2019;9:2045894019841978. doi: 10.1177/2045894019841978. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

