Time-Dependent Material Properties of Aging Biomolecular Condensates from Different Viscoelasticity Measurements in Molecular Dynamics Simulations
- PMID: 37194953
- PMCID: PMC10226127
- DOI: 10.1021/acs.jpcb.3c01292
Time-Dependent Material Properties of Aging Biomolecular Condensates from Different Viscoelasticity Measurements in Molecular Dynamics Simulations
Abstract
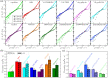
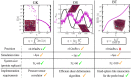
Biomolecular condensates are important contributors to the internal organization of the cell material. While initially described as liquid-like droplets, the term biomolecular condensates is now used to describe a diversity of condensed phase assemblies with material properties extending from low to high viscous liquids, gels, and even glasses. Because the material properties of condensates are determined by the intrinsic behavior of their molecules, characterizing such properties is integral to rationalizing the molecular mechanisms that dictate their functions and roles in health and disease. Here, we apply and compare three distinct computational methods to measure the viscoelasticity of biomolecular condensates in molecular simulations. These methods are the Green-Kubo (GK) relation, the oscillatory shear (OS) technique, and the bead tracking (BT) method. We find that, although all of these methods provide consistent results for the viscosity of the condensates, the GK and OS techniques outperform the BT method in terms of computational efficiency and statistical uncertainty. We thus apply the GK and OS techniques for a set of 12 different protein/RNA systems using a sequence-dependent coarse-grained model. Our results reveal a strong correlation between condensate viscosity and density, as well as with protein/RNA length and the number of stickers vs spacers in the amino acid protein sequence. Moreover, we couple the GK and the OS technique to nonequilibrium molecular dynamics simulations that mimic the progressive liquid-to-gel transition of protein condensates due to the accumulation of interprotein β-sheets. We compare the behavior of three different protein condensates, i.e., those formed by either hnRNPA1, FUS, or TDP-43 proteins, whose liquid-to-gel transitions are associated with the onset of amyotrophic lateral sclerosis and frontotemporal dementia. We find that both the GK and OS techniques successfully predict the transition from functional liquid-like behavior to kinetically arrested states once the network of interprotein β-sheets has percolated through the condensates. Overall, our work provides a comparison of different modeling rheological techniques to assess the viscosity of biomolecular condensates, a critical magnitude that provides information on the behavior of biomolecules inside condensates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Protein structural transitions critically transform the network connectivity and viscoelasticity of RNA-binding protein condensates but RNA can prevent it.Nat Commun. 2022 Sep 29;13(1):5717. doi: 10.1038/s41467-022-32874-0. Nat Commun. 2022. PMID: 36175408 Free PMC article.
-
Aging can transform single-component protein condensates into multiphase architectures.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2119800119. doi: 10.1073/pnas.2119800119. Epub 2022 Jun 21. Proc Natl Acad Sci U S A. 2022. PMID: 35727989 Free PMC article.
-
Surfactants or scaffolds? RNAs of varying lengths control the thermodynamic stability of condensates differently.Biophys J. 2023 Jul 25;122(14):2973-2987. doi: 10.1016/j.bpj.2023.03.006. Epub 2023 Mar 6. Biophys J. 2023. PMID: 36883003 Free PMC article.
-
Toward Predictive Coarse-Grained Simulations of Biomolecular Condensates.Biochemistry. 2025 Apr 15;64(8):1750-1761. doi: 10.1021/acs.biochem.4c00737. Epub 2025 Apr 2. Biochemistry. 2025. PMID: 40172489 Review.
-
Different states and the associated fates of biomolecular condensates.Essays Biochem. 2022 Dec 16;66(7):849-862. doi: 10.1042/EBC20220054. Essays Biochem. 2022. PMID: 36350032 Free PMC article. Review.
Cited by
-
Design and Characterization of DNA-Driven Condensates: Regulating Topology, Mechanical Properties, and Immunorecognition.ACS Appl Mater Interfaces. 2025 Apr 16;17(15):22322-22336. doi: 10.1021/acsami.5c00428. Epub 2025 Apr 1. ACS Appl Mater Interfaces. 2025. PMID: 40168179 Free PMC article.
-
Fundamental Aspects of Phase-Separated Biomolecular Condensates.Chem Rev. 2024 Jul 10;124(13):8550-8595. doi: 10.1021/acs.chemrev.4c00138. Epub 2024 Jun 17. Chem Rev. 2024. PMID: 38885177 Free PMC article. Review.
-
Coiled-coil domains are sufficient to drive liquid-liquid phase separation in protein models.Biophys J. 2024 Mar 19;123(6):703-717. doi: 10.1016/j.bpj.2024.02.007. Epub 2024 Feb 15. Biophys J. 2024. PMID: 38356260 Free PMC article.
-
Chemically Informed Coarse-Graining of Electrostatic Forces in Charge-Rich Biomolecular Condensates.ACS Cent Sci. 2025 Feb 11;11(2):302-321. doi: 10.1021/acscentsci.4c01617. eCollection 2025 Feb 26. ACS Cent Sci. 2025. PMID: 40028356 Free PMC article.
-
Sequence-encoded Spatiotemporal Dependence of Viscoelasticity of Protein Condensates Using Computational Microrheology.bioRxiv [Preprint]. 2024 Aug 16:2024.08.13.607792. doi: 10.1101/2024.08.13.607792. bioRxiv. 2024. Update in: JACS Au. 2024 Nov 11;4(11):4394-4405. doi: 10.1021/jacsau.4c00740. PMID: 39185151 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

