Implications of Protein Interaction in the Speciation of Potential VIVO-Pyridinone Drugs
- PMID: 37195003
- PMCID: PMC10230503
- DOI: 10.1021/acs.inorgchem.3c01041
Implications of Protein Interaction in the Speciation of Potential VIVO-Pyridinone Drugs
Abstract
Vanadium complexes (VCs) are promising agents for the treatment, among others, of diabetes and cancer. The development of vanadium-based drugs is mainly limited by a scarce knowledge of the active species in the target organs, which is often determined by the interaction of VCs with biological macromolecules like proteins. Here, we have studied the binding of [VIVO(empp)2] (where Hempp is 1-methyl-2-ethyl-3-hydroxy-4(1H)-pyridinone), an antidiabetic and anticancer VC, with the model protein hen egg white lysozyme (HEWL) by electrospray ionization-mass spectrometry (ESI-MS), electron paramagnetic resonance (EPR), and X-ray crystallography. ESI-MS and EPR techniques reveal that, in aqueous solution, both the species [VIVO(empp)2] and [VIVO(empp)(H2O)]+, derived from the first one upon the loss of a empp(-) ligand, interact with HEWL. Crystallographic data, collected under different experimental conditions, show covalent binding of [VIVO(empp)(H2O)]+ to the side chain of Asp48, and noncovalent binding of cis-[VIVO(empp)2(H2O)], [VIVO(empp)(H2O)]+, [VIVO(empp)(H2O)2]+, and of an unusual trinuclear oxidovanadium(V) complex, [VV3O6(empp)3(H2O)], with accessible sites on the protein surface. The possibility of covalent and noncovalent binding with different strength and of interaction with various sites favor the formation of adducts with the multiple binding of vanadium moieties, allowing the transport in blood and cellular fluids of more than one metal-containing species with a possible amplification of the biological effects.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

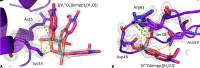


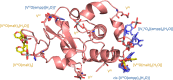
Similar articles
-
Interaction of Vanadium Complexes with Proteins: Revisiting the Reported Structures in the Protein Data Bank (PDB) since 2015.Molecules. 2023 Sep 9;28(18):6538. doi: 10.3390/molecules28186538. Molecules. 2023. PMID: 37764313 Free PMC article. Review.
-
ESI-MS Study of the Interaction of Potential Oxidovanadium(IV) Drugs and Amavadin with Model Proteins.Inorg Chem. 2020 Jul 20;59(14):9739-9755. doi: 10.1021/acs.inorgchem.0c00969. Epub 2020 Jun 25. Inorg Chem. 2020. PMID: 32585093 Free PMC article.
-
Protein-Protein Stabilization in VIVO/8-Hydroxyquinoline-Lysozyme Adducts.Chemistry. 2024 Oct 1;30(55):e202401712. doi: 10.1002/chem.202401712. Epub 2024 Sep 16. Chemistry. 2024. PMID: 38923243
-
Multiple and Variable Binding of Pharmacologically Active Bis(maltolato)oxidovanadium(IV) to Lysozyme.Inorg Chem. 2022 Oct 17;61(41):16458-16467. doi: 10.1021/acs.inorgchem.2c02690. Epub 2022 Oct 7. Inorg Chem. 2022. PMID: 36205235 Free PMC article.
-
Electrospray ionization mass spectrometry of oligonucleotide complexes with drugs, metals, and proteins.Mass Spectrom Rev. 2001 Mar-Apr;20(2):61-87. doi: 10.1002/mas.1003. Mass Spectrom Rev. 2001. PMID: 11455562 Review.
Cited by
-
Speciation and structural transformation of a VV-malate complex in the absence and in the presence of a protein: from a dinuclear species to decavanadate.Inorg Chem Front. 2025 Jul 21. doi: 10.1039/d5qi01384d. Online ahead of print. Inorg Chem Front. 2025. PMID: 40757088 Free PMC article.
-
In-Depth Mass Spectrometry Study of Vanadium(IV) Complexes with Model Peptides.Inorg Chem. 2024 Sep 23;63(38):17785-17796. doi: 10.1021/acs.inorgchem.4c02683. Epub 2024 Sep 12. Inorg Chem. 2024. PMID: 39264738 Free PMC article.
-
Interaction of Vanadium Complexes with Proteins: Revisiting the Reported Structures in the Protein Data Bank (PDB) since 2015.Molecules. 2023 Sep 9;28(18):6538. doi: 10.3390/molecules28186538. Molecules. 2023. PMID: 37764313 Free PMC article. Review.
-
Optimizing Therapeutics for Intratumoral Cancer Treatments: Antiproliferative Vanadium Complexes in Glioblastoma.Int J Mol Sci. 2025 Jan 24;26(3):994. doi: 10.3390/ijms26030994. Int J Mol Sci. 2025. PMID: 39940763 Free PMC article.
References
-
- Barry N. P. E.; Sadler P. J. Exploration of the medical periodic table: towards new targets. Chem. Commun. 2013, 49, 5106–5131. 10.1039/c3cc41143e. - DOI - PubMed
- Medici S.; Peana M.; Nurchi V. M.; Lachowicz J. I.; Crisponi G.; Zoroddu M. A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. 10.1016/j.ccr.2014.08.002. - DOI
- Metal-based Anticancer Agents. Casini A.; Anne V.; Meier-Menches S. M., Eds.; RSC: Croydon, England, 2019.
- Anthony E. J.; Bolitho E. M.; Bridgewater H. E.; Carter O. W. L.; Donnelly J. M.; Imberti C.; Lant E. C.; Lermyte F.; Needham R. J.; Palau M.; Sadler P. J.; Shi H.; Wang F.-X.; Zhang W.-Y.; Zhang Z. Metallodrugs are unique: opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. 10.1039/D0SC04082G. - DOI - PMC - PubMed
- Frei A.; Zuegg J.; Elliott A. G.; Baker M.; Braese S.; Brown C.; Chen F.; G Dowson C.; Dujardin G.; Jung N.; King A. P.; Mansour A. M.; Massi M.; Moat J.; Mohamed H. A.; Renfrew A. K.; Rutledge P. J.; Sadler P. J.; Todd M. H.; Willans C. E.; Wilson J. J.; Cooper M. A.; Blaskovich M. A. T. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. 10.1039/C9SC06460E. - DOI - PMC - PubMed
- Yousuf I.; Bashir M.; Arjmand F.; Tabassum S. Advancement of metal compounds as therapeutic and diagnostic metallodrugs: Current frontiers and future perspectives. Coord. Chem. Rev. 2021, 445, 214104.10.1016/j.ccr.2021.214104. - DOI
- Fotopoulou E.; Titilas I.; Ronconi L. Metallodrugs as Anticancer Chemotherapeutics and Diagnostic Agents: A Critical Patent Review (2010–2020). Recent Pat. Anti-Cancer Drug Discovery 2022, 17, 42–54. 10.2174/1574892816666210907101146. - DOI - PubMed
- Miranda V. M. Medicinal inorganic chemistry: an updated review on the status of metallodrugs and prominent metallodrug candidates. Rev. Inorg. Chem. 2022, 42, 29–52. 10.1515/revic-2020-0030. - DOI
-
- Levina A.; Crans D. C.; Lay P. A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. 10.1016/j.ccr.2017.01.002. - DOI
- Pessoa J. C.; Correia I. Misinterpretations in Evaluating Interactions of Vanadium Complexes with Proteins and Other Biological Targets. Inorganics 2021, 9, 17.10.3390/inorganics9020017. - DOI
-
- Pessoa J. C.; Etcheverry S.; Gambino D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. 10.1016/j.ccr.2014.12.002. - DOI - PMC - PubMed
- Kioseoglou E.; Petanidis S.; Gabriel C.; Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301-302, 87–105. 10.1016/j.ccr.2015.03.010. - DOI
- Rehder D. Perspectives for vanadium in health issues. Future Med. Chem. 2016, 8, 325–338. 10.4155/fmc.15.187. - DOI - PubMed
- Leon I. E.; Cadavid-Vargas J. F.; Di Virgilio A. L.; Etcheverry S. B. Vanadium, ruthenium and copper compounds: a new class of nonplatinum metallodrugs with anticancer activity. Curr. Med. Chem. 2017, 24, 112–148. 10.2174/0929867323666160824162546. - DOI - PubMed
- Crans D. C.; Yang L.; Haase A.; Yang X.. Health Benefits of Vanadium and Its Potential as an Anticancer Agent, Met. Ions Life Sci. In Metallo-Drugs Development & Action of Anticancer Agents ;Sigel A., Sigel H., Freisinger E., Sigel R. K. O., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2018; Vol. 18, pp 251–279. - PubMed
- Crans D. C.; LaRee H.; Cardiff G.; Posner B. I.. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver P. L., Ed.; De Gruyter GmbH: Berlin, 2019; pp 203–230. - PubMed
- Treviño S.; Díaz A.; Sánchez-Lara E.; Sanchez-Gaytan B. L.; Perez-Aguilar J. M.; González-Vergara E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. 10.1007/s12011-018-1540-6. - DOI - PMC - PubMed
- Treviño S.; Diaz A. Vanadium and insulin: Partners in metabolic regulation. J. Inorg. Biochem. 2020, 208, 111094.10.1016/j.jinorgbio.2020.111094. - DOI - PubMed
- Aureliano M.; Gumerova N. I.; Sciortino G.; Garribba E.; Rompel A.; Crans D. C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143.10.1016/j.ccr.2021.214143. - DOI
- Selvaraj S.; Krishnan U. M. Vanadium–Flavonoid Complexes: A Promising Class of Molecules for Therapeutic Applications. J. Med. Chem. 2021, 64, 12435–12452. 10.1021/acs.jmedchem.1c00405. - DOI - PubMed
- Amante C.; De Sousa-Coelho A. L.; Aureliano M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828.10.3390/met11050828. - DOI
- Sharfalddin A. A.; Al-Younis I. M.; Mohammed H. A.; Dhahri M.; Mouffouk F.; Abu Ali H.; Anwar M. J.; Qureshi K. A.; Hussien M. A.; Alghrably M.; Jaremko M.; Alasmael N.; Lachowicz J. I.; Emwas A.-H. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244.10.3390/inorganics10120244. - DOI
- Aureliano M.; Gumerova N. I.; Sciortino G.; Garribba E.; McLauchlan C. C.; Rompel A.; Crans D. C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344.10.1016/j.ccr.2021.214344. - DOI
-
- Thompson K. H.; Lichter J.; LeBel C.; Scaife M. C.; McNeill J. H.; Orvig C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. 10.1016/j.jinorgbio.2008.12.003. - DOI - PubMed
- Thompson K. H.; Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. 10.1016/j.jinorgbio.2006.08.016. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

