American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing
- PMID: 37195076
- PMCID: PMC10709681
- DOI: 10.1182/bloodadvances.2023010177
American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing
Abstract
Hereditary and acquired thrombophilia are risk factors for venous thromboembolism (VTE). Whether testing helps guide management decisions is controversial. These evidence-based guidelines from the American Society of Hematology (ASH) intend to support decision making about thrombophilia testing. ASH formed a multidisciplinary guideline panel covering clinical and methodological expertise and minimizing bias from conflicts of interest. The McMaster University GRADE Centre provided logistical support, performed systematic reviews, and created evidence profiles and evidence-to-decision tables. The Grading of Recommendations Assessment, Development, and Evaluation approach (GRADE) was used. Recommendations were subject to public comment. The panel agreed on 23 recommendations regarding thrombophilia testing and associated management. Nearly all recommendations are based on very low certainty in the evidence due to modeling assumptions. The panel issued a strong recommendation against testing the general population before starting combined oral contraceptives (COCs) and conditional recommendations for thrombophilia testing in the following scenarios: (a) patients with VTE associated with nonsurgical major transient or hormonal risk factors; (b) patients with cerebral or splanchnic venous thrombosis, in settings where anticoagulation would otherwise be discontinued; (c) individuals with a family history of antithrombin, protein C, or protein S deficiency when considering thromboprophylaxis for minor provoking risk factors and for guidance to avoid COCs/hormone replacement therapy; (d) pregnant women with a family history of high-risk thrombophilia types; and (e) patients with cancer at low or intermediate risk of thrombosis and with a family history of VTE. For all other questions, the panel provided conditional recommendations against testing for thrombophilia.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: All authors were members of either the guideline panel or of the systematic review team. Consequently, they completed a disclosure-of-interest form, which was reviewed by ASH and is available as Supplements 2 and 3.
Figures
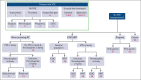
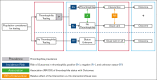
Comment in
-
Commentary on the 2023 ASH guidelines for thrombophilia testing in venous thromboembolism.Blood Adv. 2023 Nov 14;7(21):6428-6429. doi: 10.1182/bloodadvances.2023011393. Blood Adv. 2023. PMID: 37581979 Free PMC article. No abstract available.
-
Thrombophilia management calculator.Blood Adv. 2024 Aug 13;8(15):3914-3916. doi: 10.1182/bloodadvances.2024013463. Blood Adv. 2024. PMID: 38759098 Free PMC article. No abstract available.
References
-
- Institute of Medicine . National Academies Press; 2011. Clinical Practice Guidelines We Can Trust. - PubMed
-
- Qaseem A, Forland F, Macbeth F, et al. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–531. - PubMed
-
- Schunemann HJ, Al-Ansary LA, Forland F, et al. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med. 2015;163(7):548–553. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

