A Multiparameter Molecular Classifier to Predict Response to Neoadjuvant Lapatinib plus Trastuzumab without Chemotherapy in HER2+ Breast Cancer
- PMID: 37195235
- PMCID: PMC10923553
- DOI: 10.1158/1078-0432.CCR-22-3753
A Multiparameter Molecular Classifier to Predict Response to Neoadjuvant Lapatinib plus Trastuzumab without Chemotherapy in HER2+ Breast Cancer
Abstract
Purpose: Clinical trials reported 25% to 30% pathologic complete response (pCR) rates in HER2+ patients with breast cancer treated with anti-HER2 therapies without chemotherapy. We hypothesize that a multiparameter classifier can identify patients with HER2-"addicted" tumors who may benefit from a chemotherapy-sparing strategy.
Experimental design: Baseline HER2+ breast cancer specimens from the TBCRC023 and PAMELA trials, which included neoadjuvant treatment with lapatinib and trastuzumab, were used. In the case of estrogen receptor-positive (ER+) tumors, endocrine therapy was also administered. HER2 protein and gene amplification (ratio), HER2-enriched (HER2-E), and PIK3CA mutation status were assessed by dual gene protein assay (GPA), research-based PAM50, and targeted DNA-sequencing. GPA cutoffs and classifier of response were constructed in TBCRC023 using a decision tree algorithm, then validated in PAMELA.
Results: In TBCRC023, 72 breast cancer specimens had GPA, PAM50, and sequencing data, of which 15 had pCR. Recursive partitioning identified cutoffs of HER2 ratio ≥ 4.6 and %3+ IHC staining ≥ 97.5%. With PAM50 and sequencing data, the model added HER2-E and PIK3CA wild-type (WT). For clinical implementation, the classifier was locked as HER2 ratio ≥ 4.5, %3+ IHC staining ≥ 90%, and PIK3CA-WT and HER2-E, yielding 55% and 94% positive (PPV) and negative (NPV) predictive values, respectively. Independent validation using 44 PAMELA cases with all three biomarkers yielded 47% PPV and 82% NPV. Importantly, our classifier's high NPV signifies its strength in accurately identifying patients who may not be good candidates for treatment deescalation.
Conclusions: Our multiparameter classifier differentially identifies patients who may benefit from HER2-targeted therapy alone from those who need chemotherapy and predicts pCR to anti-HER2 therapy alone comparable with chemotherapy plus dual anti-HER2 therapy in unselected patients.
©2023 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interests
All remaining authors have declared no conflicts of interests
Figures


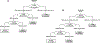
References
-
- Arpino G, Gutierrez C, Weiss H et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 2007; 99: 694–705. - PubMed
-
- Ritter CA, Perez-Torres M, Rinehart C et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 2007; 13: 4909–4919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

