[99mTc]-labelled anti-Programmed Death-Ligand 1 single-domain antibody SPECT/CT: a novel imaging biomarker for myocardial PD-L1 expression
- PMID: 37195370
- PMCID: PMC10192461
- DOI: 10.1186/s13550-023-00990-7
[99mTc]-labelled anti-Programmed Death-Ligand 1 single-domain antibody SPECT/CT: a novel imaging biomarker for myocardial PD-L1 expression
Abstract
Background: Myocardial programmed death-ligand 1 (PD-L1) expression is implicated in immune checkpoint inhibitor (ICI)-associated myocarditis. Measurement of myocardial PD-L1 expression may have potential use as a mechanistic and predictive biomarker. The aim of this study was to determine non-invasive assessment of myocardial PD-L1 expression using [99mTc]-labelled anti-PD-L1 single-domain antibody (NM-01) SPECT/CT.
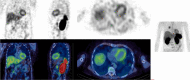
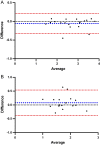
Methods: Thoracic [99mTc]NM-01 SPECT/CT was performed in lung cancer patients (n = 10) at baseline and 9-weeks following anti-programmed cell death protein 1 (PD-1) therapy. Baseline and 9-week left ventricular and right ventricular to blood pool ratios (LVmax:BP) and (RVmax:BP) were measured. LVmax was compared to background skeletal muscle (musclemax). Intra-rater reliability was determined by intraclass correlation coefficient (ICC) and Bland-Altman analysis.
Results: Mean LVmax:BP values were 2.76 ± 0.67 at baseline vs 2.55 ± 0.77 at 9 weeks (p = 0.42). Mean RVmax:BP was 1.82 ± 0.32 at baseline vs 1.76 ± 0.45 at 9 weeks (p = 0.67). Myocardial PD-L1 expression was at least threefold greater than skeletal muscle at baseline for the LV (LVmax to musclemax 3.71 ± 0.77 vs 0.98 ± 0.20 (p < 0.001)) and at least twofold for the RV (LVmax to musclemax 2.49 ± 0.63 vs 0.98 ± 0.20 (p < 0.001)). There was excellent intra-rater reliability for LVmax:BP with ICC 0.99 (95% confidence interval 0.94-0.99, p < 0.001), mean bias -0.05 ± 0.14 (95% limits of agreement -0.32 to 0.21). There were no major adverse cardiovascular events or myocarditis during follow-up.
Conclusion: This study is the first to report PD-L1 expression of the heart that can be quantified non-invasively without invasive myocardial biopsy, with high reliability and specificity. This technique can be applied to investigate myocardial PD-L1 expression in ICI-associated myocarditis and cardiomyopathies. Clinical trial registration PD-L1 Expression in Cancer (PECan) study (NCT04436406). https://clinicaltrials.gov/ct2/show/NCT04436406 June 18th, 2020.
Keywords: Immune checkpoint inhibitor; PD-L1 expression; SPECT-CT.
© 2023. The Author(s).
Conflict of interest statement
DJH has received speaker fees and/or travel reimbursement from Novartis, Pfizer and BMS; DJH and GJRC have received research funding via institution from Nanomab Technology (UK) Ltd; GJRC has received consultation fees and radiotracer for research from Nanomab Technology (UK) Ltd. GC and HHT are employees of Nanomab Technology (UK) Ltd. All other authors have no relevant disclosures.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

