Psychometric evaluation of the Symptoms of Infection with Coronavirus-19 (SIC): results from a cross-sectional study and a phase 3 clinical trial
- PMID: 37195456
- PMCID: PMC10189706
- DOI: 10.1186/s41687-023-00581-z
Psychometric evaluation of the Symptoms of Infection with Coronavirus-19 (SIC): results from a cross-sectional study and a phase 3 clinical trial
Abstract
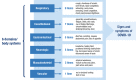
Background: The Symptoms of Infection with Coronavirus-19 (SIC) is a 30-item patient-reported outcome (PRO) measure scored by body system composites to assess signs/symptoms of coronavirus disease 2019 (COVID-19). In addition to cross-sectional and longitudinal psychometric evaluations, qualitative exit interviews were conducted to support the content validity of the SIC.
Methods: In a cross-sectional study, adults diagnosed with COVID-19 in the United States completed the web-based SIC and additional PRO measures. A subset was invited to participate in phone-based exit interviews. Longitudinal psychometric properties were assessed in ENSEMBLE2, a multinational, randomized, double-blind, placebo-controlled, phase 3 trial of the Ad26.COV2.S COVID-19 vaccine. Psychometric properties evaluated included structure, scoring, reliability, construct validity, discriminating ability, responsiveness, and meaningful change thresholds of SIC items and composite scores.
Results: In the cross-sectional study, 152 participants completed the SIC (mean age, 51.0 ± 18.6 years) and 20 completed follow-up interviews. Fatigue (77.6%), feeling unwell (65.8%), and cough (60.5%) were symptoms most frequently reported. SIC inter-item correlations were all positive and mostly moderate (r ≥ 0.3) and statistically significant. SIC items and Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) scores correlated as hypothesized (all r ≥ 0.32). Internal consistency reliabilities of all SIC composite scores were satisfactory (Cronbach's alpha, 0.69-0.91). SIC composite scores correlated moderately (r = 0.30-0.49) to strongly (r ≥ 0.50) with PROMIS-29 scores and Patient Global Impression of Severity (PGIS) ratings (all P < 0.01). A variety of signs/symptoms were cited in exit interviews, and participants considered the SIC straightforward, comprehensive, and easy to use. From ENSEMBLE2, 183 participants with laboratory-confirmed moderate to severe/critical COVID-19 were included (51.5 ± 14.8 years). Strong test-retest reliabilities were observed for most SIC composite scores (intraclass correlations ≥ 0.60). Statistically significant differences across PGIS severity levels were found for all but 1 composite score, supporting known-groups validity. All SIC composite scores demonstrated responsiveness based on changes in PGIS.
Conclusions: The psychometric evaluations provided strong evidence for the reliability and validity of the SIC for measuring COVID-19 symptoms, supporting its use in vaccine and treatment trials. In exit interviews, participants described a broad range of signs/symptoms consistent with previous research, further supporting the content validity and format of the SIC.
Keywords: COVID-19; Patient-reported outcome; Psychometric evaluation; Reliability; Validity.
Plain language summary
Coronavirus disease 2019 (COVID-19) is a serious disease that continues to evolve globally. Researchers developed the Symptoms of Infection with Coronavirus-19 (SIC), a 30-item questionnaire designed for patients to report signs and symptoms of COVID-19. In this study, the researchers formally analyzed how well the SIC measures the patient experience with COVID-19, using survey and clinical trial data as well as telephone interviews. Adults with COVID-19 and at least 2 bothersome symptoms completed the web-based survey, and some of these individuals also participated in in-depth interviews. Participants in a clinical trial for a COVID-19 vaccine also completed the SIC measure. The SIC was compared with other commonly used questionnaires that evaluate patient experience. The most commonly reported symptoms of COVID-19 were fatigue, feeling unwell, cough, weakness, and headache. The items for individual symptoms (e.g., “cough”) and combined scores for body systems (e.g., “respiratory system”) performed well in statistical analyses. Participants found the SIC to be straightforward, comprehensive, and easy to use. The SIC may prove useful in the future for vaccine and treatment trials for COVID-19.
© 2023. The Author(s).
Conflict of interest statement
VW, CR, SF, MM, SL, and SY are employees of RTI Health Solutions, which received research funding from Janssen during the conduct of this study. JM was an employee of RTI Health Solutions at the time of the study. EKHC, EGK, and PM are employees of Janssen Global Services, LLC, and hold stock in Johnson & Johnson. JSt, YL, and KM were employees of Janssen at the time of the study. JSa is an employee of Janssen Research & Development, LLC, and holds stock in Johnson & Johnson. IvD is an employee of Johnson & Johnson. AFS is a consultant to Janssen Global Services, LLC.
Figures


References
-
- Centers for Disease Control and Prevention (CDC) (2021) COVID data tracker. COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-... Accessed September 16, 2022
-
- World Health Organization (WHO) (2021) WHO coronavirus (COVID-19) dashboard 2021. https://covid19.who.int/. Accessed September 16, 2022
-
- World Health Organization (WHO) (2022) Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed February 12, 2023
-
- Centers for Disease Control and Prevention (CDC) (2022) What you need to know about variants. https://www.cdc.gov/coronavirus/2019-ncov/variants/about-variants.html. Accessed August 3, 2022
-
- Centers for Disease Control and Prevention (CDC) (2022) SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed August 4, 2022
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

