Effectiveness of S-1-Based Chemoradiotherapy in Patients 70 Years and Older With Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial
- PMID: 37195667
- PMCID: PMC10193185
- DOI: 10.1001/jamanetworkopen.2023.12625
Effectiveness of S-1-Based Chemoradiotherapy in Patients 70 Years and Older With Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial
Abstract
Importance: Double-agent intravenous chemotherapy concurrent with radiotherapy is the standard of care for patients with inoperable esophageal cancer. However, patients tend to tolerate intravenous chemotherapy less well with age and comorbidities. It is essential to find a better treatment modality that improves survival outcomes without reducing the quality of life.
Objective: To evaluate the effectiveness of simultaneous integrated boost radiotherapy (SIB-RT) with concurrent and consolidated oral S-1 chemotherapy for patients aged 70 years and older with inoperable esophageal squamous cell carcinoma (ESCC).
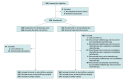
Design, setting, and participants: This multicenter, phase III randomized clinical trial was conducted between March 2017 and April 2020 in 10 centers in China. Patients with inoperable, locally advanced, clinical stage II to IV ESCC were enrolled and randomized to receive SIB-RT concurrent with and followed by oral S-1 chemotherapy (CRTCT group) or SIB-RT alone (RT group). Data analysis was completed on March 22, 2022.
Interventions: In both groups, the planning gross tumor volume was administered with radiation dose of 59.92 Gy and the planning target volume was administered with radiation dose of 50.4 Gy, in 28 fractions each. In the CRTCT group, concurrent S-1 was administered on radiotherapy days, and consolidated S-1 was administered at 4 to 8 weeks after SIB-RT.
Main outcomes and measures: The primary end point was overall survival (OS) of the intent-to-treat population. Secondary end points were progression-free survival (PFS) and toxicity profile.
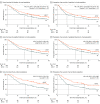
Results: A total of 330 patients (median [IQR] age, 75.5 [72-79] years; 220 [66.7%] male patients) were included, with 146 patients randomized to the RT group and 184 randomized to the CRTCT group. A total of 107 patients (73.3%) in the RT group and 121 patients (67.9%) in the CRTCT group were clinically diagnosed with stage III to IV disease. At the time of analysis of the 330 patients in the intent-to treat-population (March 22, 2022), OS was improved in the CRTCT group compared with the RT group at 1 year (72.2% vs 62.3%) and 3 years (46.2% vs 33.9%; log-rank P = .02). PFS was similarly improved in the CRTCT group compared with the RT group at 1 year (60.8% vs 49.3%) and 3 years (37.3% vs 27.9%; log-rank P = .04). There was no significant difference in the incidence of treatment-related toxic effects higher than grade 3 between the 2 groups. Grade 5 toxic effects occurred in each group, including 1 patient who experienced myelosuppression and 4 patients with pneumonitis in the RT group and 3 patients with pneumonitis and 2 patients with fever in the CRTCT group.
Conclusions and relevance: These findings suggest that oral S-1 chemotherapy administered with SIB-RT should be considered as an alternative treatment option for patients aged 70 years and older with inoperable ESCC, since it improved survival outcomes without additional treatment-related toxic effects compared with SIB-RT alone.
Trial registration: ClinicalTrials.gov Identifier: NCT02979691.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

