Vigorous Exercise in Patients With Hypertrophic Cardiomyopathy
- PMID: 37195701
- PMCID: PMC10193262
- DOI: 10.1001/jamacardio.2023.1042
Vigorous Exercise in Patients With Hypertrophic Cardiomyopathy
Abstract
Importance: Whether vigorous intensity exercise is associated with an increase in risk of ventricular arrhythmias in individuals with hypertrophic cardiomyopathy (HCM) is unknown.
Objective: To determine whether engagement in vigorous exercise is associated with increased risk for ventricular arrhythmias and/or mortality in individuals with HCM. The a priori hypothesis was that participants engaging in vigorous activity were not more likely to have an arrhythmic event or die than those who reported nonvigorous activity.
Design, setting, and participants: This was an investigator-initiated, prospective cohort study. Participants were enrolled from May 18, 2015, to April 25, 2019, with completion in February 28, 2022. Participants were categorized according to self-reported levels of physical activity: sedentary, moderate, or vigorous-intensity exercise. This was a multicenter, observational registry with recruitment at 42 high-volume HCM centers in the US and internationally; patients could also self-enroll through the central site. Individuals aged 8 to 60 years diagnosed with HCM or genotype positive without left ventricular hypertrophy (phenotype negative) without conditions precluding exercise were enrolled.
Exposures: Amount and intensity of physical activity.
Main outcomes and measures: The primary prespecified composite end point included death, resuscitated sudden cardiac arrest, arrhythmic syncope, and appropriate shock from an implantable cardioverter defibrillator. All outcome events were adjudicated by an events committee blinded to the patient's exercise category.
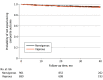
Results: Among the 1660 total participants (mean [SD] age, 39 [15] years; 996 male [60%]), 252 (15%) were classified as sedentary, and 709 (43%) participated in moderate exercise. Among the 699 individuals (42%) who participated in vigorous-intensity exercise, 259 (37%) participated competitively. A total of 77 individuals (4.6%) reached the composite end point. These individuals included 44 (4.6%) of those classified as nonvigorous and 33 (4.7%) of those classified as vigorous, with corresponding rates of 15.3 and 15.9 per 1000 person-years, respectively. In multivariate Cox regression analysis of the primary composite end point, individuals engaging in vigorous exercise did not experience a higher rate of events compared with the nonvigorous group with an adjusted hazard ratio of 1.01. The upper 95% 1-sided confidence level was 1.48, which was below the prespecified boundary of 1.5 for noninferiority.
Conclusions and relevance: Results of this cohort study suggest that among individuals with HCM or those who are genotype positive/phenotype negative and are treated in experienced centers, those exercising vigorously did not experience a higher rate of death or life-threatening arrhythmias than those exercising moderately or those who were sedentary. These data may inform discussion between the patient and their expert clinician around exercise participation.
Conflict of interest statement
Figures

References
-
- Maron BJ, Gaffney FA, Jeresaty RM, McKenna WJ, Miller WW. Cardiovascular abnormalities in the athlete: recommendations regarding eligibility for competition—task force III: Hypertrophic cardiomyopathy, other myopericardial diseases and mitral valve prolapse. J Am Coll Cardiol. 1985;6(6):1215-1217. doi: 10.1016/S0735-1097(85)80203-5 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

