B Cell Chronic Lymphocytic Leukemia Development in Mice with Chronic Lung Exposure to Coccidioides Fungal Arthroconidia
- PMID: 37195872
- PMCID: PMC10579974
- DOI: 10.4049/immunohorizons.2300013
B Cell Chronic Lymphocytic Leukemia Development in Mice with Chronic Lung Exposure to Coccidioides Fungal Arthroconidia
Abstract
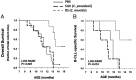
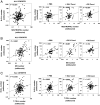
Links between repeated microbial infections and B cell chronic lymphocytic leukemia (B-CLL) have been proposed but not tested directly. This study examines how prolonged exposure to a human fungal pathogen impacts B-CLL development in Eµ-hTCL1-transgenic mice. Monthly lung exposure to inactivated Coccidioides arthroconidia, agents of Valley fever, altered leukemia development in a species-specific manner, with Coccidioides posadasii hastening B-CLL diagnosis/progression in a fraction of mice and Coccidioides immitis delaying aggressive B-CLL development, despite fostering more rapid monoclonal B cell lymphocytosis. Overall survival did not differ significantly between control and C. posadasii-treated cohorts but was significantly extended in C. immitis-exposed mice. In vivo doubling time analyses of pooled B-CLL showed no difference in growth rates of early and late leukemias. However, within C. immitis-treated mice, B-CLL manifests longer doubling times, as compared with B-CLL in control or C. posadasii-treated mice, and/or evidence of clonal contraction over time. Through linear regression, positive relationships were noted between circulating levels of CD5+/B220low B cells and hematopoietic cells previously linked to B-CLL growth, albeit in a cohort-specific manner. Neutrophils were positively linked to accelerated growth in mice exposed to either Coccidioides species, but not in control mice. Conversely, only C. posadasii-exposed and control cohorts displayed positive links between CD5+/B220low B cell frequency and abundance of M2 anti-inflammatory monocytes and T cells. The current study provides evidence that chronic lung exposure to fungal arthroconidia affects B-CLL development in a manner dependent on fungal genotype. Correlative studies suggest that fungal species differences in the modulation of nonleukemic hematopoietic cells are involved.
Copyright © 2023 The Authors.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures







References
-
- Scarfò, L., Ghia P.. 2016. What does it mean I have a monoclonal B-cell lymphocytosis?: recent insights and new challenges. Semin. Oncol. 43: 201–208. - PubMed
-
- Messmer, B. T., Albesiano E., Efremov D. G., Ghiotto F., Allen S. L., Kolitz J., Foa R., Damle R. N., Fais F., Messmer D., et al. 2004. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J. Exp. Med. 200: 519–525. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

