Integrated post-genomic cell wall analysis reveals floating biofilm formation associated with high expression of flocculins in the pathogen Pichia kudriavzevii
- PMID: 37196016
- PMCID: PMC10228781
- DOI: 10.1371/journal.ppat.1011158
Integrated post-genomic cell wall analysis reveals floating biofilm formation associated with high expression of flocculins in the pathogen Pichia kudriavzevii
Abstract
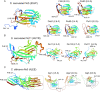
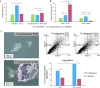
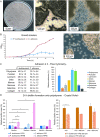
The pathogenic yeast Pichia kudriavzevii, previously known as Candida krusei, is more distantly related to Candida albicans than clinically relevant CTG-clade Candida species. Its cell wall, a dynamic organelle that is the first point of interaction between pathogen and host, is relatively understudied, and its wall proteome remains unidentified to date. Here, we present an integrated study of the cell wall in P. kudriavzevii. Our comparative genomic studies and experimental data indicate that the general structure of the cell wall in P. kudriavzevii is similar to Saccharomyces cerevisiae and C. albicans and is comprised of β-1,3-glucan, β-1,6-glucan, chitin, and mannoproteins. However, some pronounced differences with C. albicans walls were observed, for instance, higher mannan and protein levels and altered protein mannosylation patterns. Further, despite absence of proteins with high sequence similarity to Candida adhesins, protein structure modeling identified eleven proteins related to flocculins/adhesins in S. cerevisiae or C. albicans. To obtain a proteomic comparison of biofilm and planktonic cells, P. kudriavzevii cells were grown to exponential phase and in static 24-h cultures. Interestingly, the 24-h static cultures of P. kudriavzevii yielded formation of floating biofilm (flor) rather than adherence to polystyrene at the bottom. The proteomic analysis of both conditions identified a total of 33 cell wall proteins. In line with a possible role in flor formation, increased abundance of flocculins, in particular Flo110, was observed in the floating biofilm compared to exponential cells. This study is the first to provide a detailed description of the cell wall in P. kudriavzevii including its cell wall proteome, and paves the way for further investigations on the importance of flor formation and flocculins in the pathogenesis of P. kudriavzevii.
Copyright: © 2023 Alvarado et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Yadav JS, Bezawada J, Yan S, Tyagi RD, Surampalli RY. Candida krusei: biotechnological potentials and concerns about its safety. Can J Microbiol. 2012;58(8):937–52. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

