Clonally expanded, thyrotoxic effector CD8+ T cells driven by IL-21 contribute to checkpoint inhibitor thyroiditis
- PMID: 37196065
- PMCID: PMC10227862
- DOI: 10.1126/scitranslmed.adg0675
Clonally expanded, thyrotoxic effector CD8+ T cells driven by IL-21 contribute to checkpoint inhibitor thyroiditis
Abstract
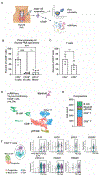
Autoimmune toxicity occurs in up to 60% of patients treated with immune checkpoint inhibitor (ICI) therapy for cancer and represents an increasing clinical challenge for expanding the use of these treatments. To date, human immunopathogenic studies of immune-related adverse events (IRAEs) have relied on sampling of circulating peripheral blood cells rather than affected tissues. Here, we directly obtained thyroid specimens from individuals with ICI-thyroiditis, one of the most common IRAEs, and compared immune infiltrates with those from individuals with spontaneous autoimmune Hashimoto's thyroiditis (HT) or no thyroid disease. Single-cell RNA sequencing revealed a dominant, clonally expanded population of thyroid-infiltrating cytotoxic CXCR6+ CD8+ T cells (effector CD8+ T cells) present in ICI-thyroiditis but not HT or healthy controls. Furthermore, we identified a crucial role for interleukin-21 (IL-21), a cytokine secreted by intrathyroidal T follicular (TFH) and T peripheral helper (TPH) cells, as a driver of these thyrotoxic effector CD8+ T cells. In the presence of IL-21, human CD8+ T cells acquired the activated effector phenotype with up-regulation of the cytotoxic molecules interferon-γ (IFN-γ) and granzyme B, increased expression of the chemokine receptor CXCR6, and thyrotoxic capacity. We validated these findings in vivo using a mouse model of IRAEs and further demonstrated that genetic deletion of IL-21 signaling protected ICI-treated mice from thyroid immune infiltration. Together, these studies reveal mechanisms and candidate therapeutic targets for individuals who develop IRAEs.
Conflict of interest statement
A.R. has received honoraria from consulting with Amgen, Bristol-Myers Squibb, Chugai, Genentech, Merck, Novartis, Roche, Sanofi and Vedanta, is or has been a member of the scientific advisory board and holds stock in Advaxis, Appia, Apricity, Arcus, Compugen, CytomX, Highlight, ImaginAb, Isoplexis, Kalthera, Kite-Gilead, Merus, PACT Pharma, Pluto, RAPT, Rgenix, Synthekine and Tango, has received research funding from Agilent and from Bristol-Myers Squibb through Stand Up to Cancer (SU2C), and patent royalties from Arsenal Bio. A.D. has consulted for Bristol-Myers Squibb, AstraZeneca, Radmetrix, Seattle Genetics, Janssen, PACT Pharma, Merck, Roche/Genetech, Exelixis, Dyania Health, and has received research funding Kite/Gilead, AstraZeneca, Roche/Genetech, BMS, Merck, Jounce Therapeutics, Infinity Pharmaceuticals, Seattle Genetics. All other authors declare that they have no competing interests.
Figures




References
-
- Wei SC, Duffy CR, Allison JP, Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018). - PubMed
-
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB, Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 4, 1721–1728 (2018). - PMC - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD, Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med 381, 1535–1546 (2019). - PubMed
-
- De Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B, A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint InhibitorsHorm. Metab. Res 51, 145–156 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

