Hypofractionated Stereotactic Re-irradiation and Anti-PDL1 Durvalumab Combination in Recurrent Glioblastoma: STERIMGLI Phase I Results
- PMID: 37196069
- PMCID: PMC10485381
- DOI: 10.1093/oncolo/oyad095
Hypofractionated Stereotactic Re-irradiation and Anti-PDL1 Durvalumab Combination in Recurrent Glioblastoma: STERIMGLI Phase I Results
Abstract
Background: Hypofractionated stereotactic radiotherapy (hFSRT) is a salvage option for recurrent glioblastoma (GB) which may synergize anti-PDL1 treatment. This phase I study evaluated the safety and the recommended phase II dose of anti-PDL1 durvalumab combined with hFSRT in patients with recurrent GB.
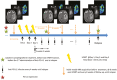
Methods: Patients were treated with 24 Gy, 8 Gy per fraction on days 1, 3, and 5 combined with the first 1500 mg Durvalumab dose on day 5, followed by infusions q4weeks until progression or for a maximum of 12 months. A standard 3 + 3 Durvalumab dose de-escalation design was used. Longitudinal lymphocytes count, cytokines analyses on plasma samples, and magnetic resonance imaging (MRI) were collected.
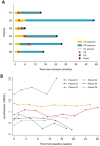
Results: Six patients were included. One dose limiting toxicity, an immune-related grade 3 vestibular neuritis related to Durvalumab, was reported. Median progression-free interval (PFI) and overall survival (OS) were 2.3 and 16.7 months, respectively. Multi-modal deep learning-based analysis including MRI, cytokines, and lymphocytes/neutrophil ratio isolated the patients presenting pseudoprogression, the longest PFI and those with the longest OS, but statistical significance cannot be established considering phase I data only.
Conclusion: Combination of hFSRT and Durvalumab in recurrent GB was well tolerated in this phase I study. These encouraging results led to an ongoing randomized phase II. (ClinicalTrials.gov Identifier: NCT02866747).
Keywords: Durvalumab; deep learning; hypofractionated stereotactic re-irradiation; phase I clinical trial; recurrent glioblastoma.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Damien Pouessel reported travel fees from AstraZeneca, Ipsen, scientific advisory board member for Pfizer, Merck, MSD, and Astellas, and honoraria from Pfizer, Merck, MSD, Janssen, and BMS. Marie Robert reported travel fees from Merck and Novartis and scientific advisory board member for Novartis and Eisai. Jean-Sebastien Frenel reported honoraria from Roche, AstraZeneca, Novartis, Daiichi, Lilly, Pfizer, Clovis, GSK, MSD, Gilead, Seagen, and Amgen. Elizabeth Cohen-Jonathan Moyal reported research funding from AstraZeneca, Novocure, Bayer, and Incyte, and scientific advisory board member and intellectual property with Novocure. The other authors indicated no financial relationships.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

