Mice with humanized livers reveal the role of hepatocyte clocks in rhythmic behavior
- PMID: 37196091
- PMCID: PMC10191436
- DOI: 10.1126/sciadv.adf2982
Mice with humanized livers reveal the role of hepatocyte clocks in rhythmic behavior
Abstract
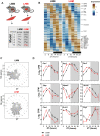
The synchronization of circadian clock depends on a central pacemaker located in the suprachiasmatic nuclei. However, the potential feedback of peripheral signals on the central clock remains poorly characterized. To explore whether peripheral organ circadian clocks may affect the central pacemaker, we used a chimeric model in which mouse hepatocytes were replaced by human hepatocytes. Liver humanization led to reprogrammed diurnal gene expression and advanced the phase of the liver circadian clock that extended to muscle and the entire rhythmic physiology. Similar to clock-deficient mice, liver-humanized mice shifted their rhythmic physiology more rapidly to the light phase under day feeding. Our results indicate that hepatocyte clocks can affect the central pacemaker and offer potential perspectives to apprehend pathologies associated with altered circadian physiology.
Figures





References
-
- M. R. Ralph, R. G. Foster, F. C. Davis, M. Menaker, Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 (1990). - PubMed
-
- S. H. Yoo, S. Yamazaki, P. L. Lowrey, K. Shimomura, C. H. Ko, E. D. Buhr, S. M. Siepka, H. K. Hong, W. J. Oh, O. J. Yoo, M. Menaker, J. S. Takahashi, PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339–5346 (2004). - PMC - PubMed
-
- F. Sinturel, P. Gos, V. Petrenko, C. Hagedorn, F. Kreppel, K. F. Storch, D. Knutti, A. Liani, C. Weitz, Y. Emmenegger, P. Franken, L. Bonacina, C. Dibner, U. Schibler, Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes Dev. 35, 329–334 (2021). - PMC - PubMed
-
- B. Kornmann, O. Schaad, H. Reinke, C. Saini, U. Schibler, Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb. Symp. Quant. Biol. 72, 319–330 (2007). - PubMed
-
- B. D. Weger, C. Gobet, F. P. A. David, F. Atger, E. Martin, N. E. Phillips, A. Charpagne, M. Weger, F. Naef, F. Gachon, Systematic analysis of differential rhythmic liver gene expression mediated by the circadian clock and feeding rhythms. Proc. Natl. Acad. Sci. U.S.A. 118, e2015803118 (2021). - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

