Mitochondrial Dynamics: Working with the Cytoskeleton and Intracellular Organelles to Mediate Mechanotransduction
- PMID: 37196113
- PMCID: PMC10529762
- DOI: 10.14336/AD.2023.0201
Mitochondrial Dynamics: Working with the Cytoskeleton and Intracellular Organelles to Mediate Mechanotransduction
Abstract
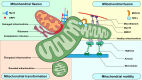
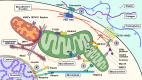
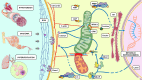
Cells are constantly exposed to various mechanical environments; therefore, it is important that they are able to sense and adapt to changes. It is known that the cytoskeleton plays a critical role in mediating and generating extra- and intracellular forces and that mitochondrial dynamics are crucial for maintaining energy homeostasis. Nevertheless, the mechanisms by which cells integrate mechanosensing, mechanotransduction, and metabolic reprogramming remain poorly understood. In this review, we first discuss the interaction between mitochondrial dynamics and cytoskeletal components, followed by the annotation of membranous organelles intimately related to mitochondrial dynamic events. Finally, we discuss the evidence supporting the participation of mitochondria in mechanotransduction and corresponding alterations in cellular energy conditions. Notable advances in bioenergetics and biomechanics suggest that the mechanotransduction system composed of mitochondria, the cytoskeletal system, and membranous organelles is regulated through mitochondrial dynamics, which may be a promising target for further investigation and precision therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mitochondria: At the crossroads between mechanobiology and cell metabolism.Biol Cell. 2023 Sep;115(9):e2300010. doi: 10.1111/boc.202300010. Epub 2023 Jul 3. Biol Cell. 2023. PMID: 37326132 Review.
-
Mechanobiology of organelles: illuminating their roles in mechanosensing and mechanotransduction.Trends Cell Biol. 2023 Dec;33(12):1049-1061. doi: 10.1016/j.tcb.2023.05.001. Epub 2023 May 24. Trends Cell Biol. 2023. PMID: 37236902 Review.
-
Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders.Neurosci Biobehav Rev. 2022 Nov;142:104892. doi: 10.1016/j.neubiorev.2022.104892. Epub 2022 Sep 28. Neurosci Biobehav Rev. 2022. PMID: 36181925 Review.
-
Mitochondrial dynamics and mitochondrial quality control.Redox Biol. 2015;4:6-13. doi: 10.1016/j.redox.2014.11.006. Epub 2014 Nov 20. Redox Biol. 2015. PMID: 25479550 Free PMC article. Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
Cited by
-
Underneath the Gut-Brain Axis in IBD-Evidence of the Non-Obvious.Int J Mol Sci. 2024 Nov 12;25(22):12125. doi: 10.3390/ijms252212125. Int J Mol Sci. 2024. PMID: 39596193 Free PMC article. Review.
-
Identification and Validation of Key Biomarkers in the Proximal Aqueous Humor Outflow Pathway.Curr Issues Mol Biol. 2025 Feb 25;47(3):147. doi: 10.3390/cimb47030147. Curr Issues Mol Biol. 2025. PMID: 40136401 Free PMC article.
-
Disulfidptosis, A Novel Cell Death Pathway: Molecular Landscape and Therapeutic Implications.Aging Dis. 2024 May 2;16(2):917-945. doi: 10.14336/AD.2024.0083. Aging Dis. 2024. PMID: 38739940 Free PMC article. Review.
-
Deciphering the intracellular forces shaping mitochondrial motion.Sci Rep. 2024 Oct 13;14(1):23914. doi: 10.1038/s41598-024-74734-5. Sci Rep. 2024. PMID: 39397143 Free PMC article.
-
The role of Mitofusin-1 and Mitofusin-2 in periodontal disease: a comprehensive review.Front Oral Health. 2025 Jan 17;6:1540178. doi: 10.3389/froh.2025.1540178. eCollection 2025. Front Oral Health. 2025. PMID: 39896143 Free PMC article. Review.
References
-
- Huang L, Yang Z, Liu R, Xiao X, Zhou C, Yin X et al. (2021). Lactoferrin promotes osteogenesis of MC3T3-E1 cells induced by mechanical strain in an extracellular signal-regulated kinase 1/2-dependent manner. Am J Orthod Dentofacial Orthop, 159:e113-21. - PubMed
-
- French AS (1992). Mechanotransduction. Ann Rev Physiol, 54:135-52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
