Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E-based Antibody-Drug Conjugate for Treatment of Nectin-4-expressing Cancers
- PMID: 37196158
- PMCID: PMC10390865
- DOI: 10.1158/1535-7163.MCT-22-0743
Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E-based Antibody-Drug Conjugate for Treatment of Nectin-4-expressing Cancers
Abstract
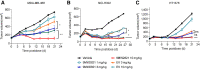
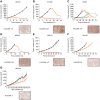
Overexpression of nectin cell adhesion protein 4 correlates with cancer progression and poor prognosis in many human malignancies. Enfortumab vedotin (EV) is the first nectin-4-targeting antibody-drug conjugate (ADC) approved by the FDA for the treatment of urothelial cancer. However, inadequate efficacy has limited progress in the treatment of other solid tumors with EV. Furthermore, ocular, pulmonary, and hematologic toxic side effects are common in nectin-4-targeted therapy, which frequently results in dose reduction and/or treatment termination. Thus, we designed a second generation nectin-4-specific drug, 9MW2821, based on interchain-disulfide drug conjugate technology. This novel drug contained a site specifically conjugated humanized antibody and the cytotoxic moiety monomethyl auristatin E. The homogenous drug-antibody ratio and novel linker chemistry of 9MW2821 increased the stability of conjugate in the systemic circulation, enabling highly efficient drug delivery and avoiding off-target toxicity. In preclinical evaluation, 9MW2821 exhibited nectin-4-specific cell binding, efficient internalization, bystander killing, and equivalent or superior antitumor activity compared with EV in both cell line-derived xenograft and patient-derived xenograft (PDX) models. In addition, 9MW2821 demonstrated a favorable safety profile; the highest nonseverely toxic dose in monkey toxicologic studies was 6 mg/kg, with milder adverse events compared with EV. Overall, 9MW2821 is a nectin-4-directed, investigational ADC based on innovative technology that endowed the drug with compelling preclinical antitumor activity and a favorable therapeutic index. The 9MW2821 ADC is being investigated in a phase I/II clinical trial (NCT05216965 and NCT05773937) in patients with advanced solid tumors.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- 1535-7163. doi: 10.1158/1535-7163.MCT-22-8-HI
References
-
- Bouleftour W, Guillot A, Magne N. The anti-nectin 4: a promising tumor cells target. a systematic review. Mol Cancer Ther 2022;21:493–501. - PubMed
-
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem 2001;276:43205–15. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

