In Vivo Sampling of Intracellular Heterogeneity of Pseudomonas putida Enables Multiobjective Optimization of Genetic Devices
- PMID: 37196337
- PMCID: PMC10278179
- DOI: 10.1021/acssynbio.3c00009
In Vivo Sampling of Intracellular Heterogeneity of Pseudomonas putida Enables Multiobjective Optimization of Genetic Devices
Abstract
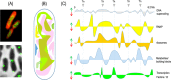
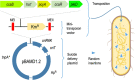
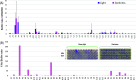
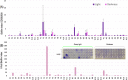
The inner physicochemical heterogeneity of bacterial cells generates three-dimensional (3D)-dependent variations of resources for effective expression of given chromosomally located genes. This fact has been exploited for adjusting the most favorable parameters for implanting a complex device for optogenetic control of biofilm formation in the soil bacterium Pseudomonas putida. To this end, a DNA segment encoding a superactive variant of the Caulobacter crescendus diguanylate cyclase PleD expressed under the control of the cyanobacterial light-responsive CcaSR system was placed in a mini-Tn5 transposon vector and randomly inserted through the chromosome of wild-type and biofilm-deficient variants of P. putida lacking the wsp gene cluster. This operation delivered a collection of clones covering a whole range of biofilm-building capacities and dynamic ranges in response to green light. Since the phenotypic output of the device depends on a large number of parameters (multiple promoters, RNA stability, translational efficacy, metabolic precursors, protein folding, etc.), we argue that random chromosomal insertions enable sampling the intracellular milieu for an optimal set of resources that deliver a preset phenotypic specification. Results thus support the notion that the context dependency can be exploited as a tool for multiobjective optimization, rather than a foe to be suppressed in Synthetic Biology constructs.
Keywords: CcaSR system; PleD; Pseudomonas; biofilm; interoperability; transposon.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Opgenorth P.; Costello Z.; Okada T.; Goyal G.; Chen Y.; Gin J.; Benites V.; de Raad M.; Northen T. R.; Deng K.; Deutsch S.; Baidoo E. E. K.; Petzold C. J.; Hillson N. J.; Garcia Martin H.; Beller H. R. Lessons from Two Design–Build–Test–Learn Cycles of Dodecanol Production in Escherichia coli Aided by Machine Learning. ACS Synth. Biol. 2019, 8, 1337–1351. 10.1021/acssynbio.9b00020. - DOI - PubMed
-
- Miskovic L.; Alff-Tuomala S.; Soh K. C.; Barth D.; Salusjärvi L.; Pitkänen J.-P.; Ruohonen L.; Penttilä M.; Hatzimanikatis V. A design–build–test cycle using modeling and experiments reveals interdependencies between upper glycolysis and xylose uptake in recombinant S. cerevisiae and improves predictive capabilities of large-scale kinetic models. Biotechnol. Biofuels 2017, 10, 166 10.1186/s13068-017-0838-5. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

