Structure, Function, and Molecular Landscapes of the Aging Retina
- PMID: 37196423
- PMCID: PMC10524587
- DOI: 10.1146/annurev-vision-112122-020950
Structure, Function, and Molecular Landscapes of the Aging Retina
Abstract
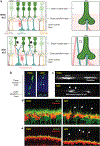
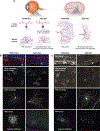
Because the central nervous system is largely nonrenewing, neurons and their synapses must be maintained over the lifetime of an individual to ensure circuit function. Age is a dominant risk factor for neural diseases, and declines in nervous system function are a common feature of aging even in the absence of disease. These alterations extend to the visual system and, in particular, to the retina. The retina is a site of clinically relevant age-related alterations but has also proven to be a uniquely approachable system for discovering principles that govern neural aging because it is well mapped, contains diverse neuron types, and is experimentally accessible. In this article, we review the structural and molecular impacts of aging on neurons within the inner and outer retina circuits. We further discuss the contribution of non-neuronal cell types and systems to retinal aging outcomes. Understanding how and why the retina ages is critical to efforts aimed at preventing age-related neural decline and restoring neural function.
Keywords: aging; neuron; retina; synapse.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

