Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals
- PMID: 37196656
- PMCID: PMC10188274
- DOI: 10.1016/j.chom.2023.05.001
Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals
Abstract
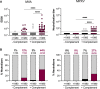
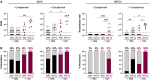
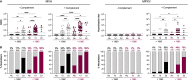
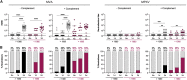
Mpox virus (MPXV) caused a multi-country outbreak in non-endemic areas in 2022. Following historic success of smallpox vaccination with vaccinia virus (VACV)-based vaccines, the third generation modified vaccinia Ankara (MVA)-based vaccine was used as prophylaxis for MPXV, but its effectiveness remains poorly characterized. Here, we applied two assays to quantify neutralizing antibodies (NAbs) in sera from control, MPXV-infected, or MVA-vaccinated individuals. Various levels of MVA NAbs were detected after infection, historic smallpox, or recent MVA vaccination. MPXV was minimally sensitive to neutralization. However, addition of complement enhanced detection of responsive individuals and NAb levels. Anti-MVA and -MPXV NAbs were observed in 94% and 82% of infected individuals, respectively, and 92% and 56% of MVA vaccinees, respectively. NAb titers were higher in individuals born before 1980, highlighting the impact of historic smallpox vaccination on humoral immunity. Altogether, our results indicate that MPXV neutralization is complement dependent and uncover mechanisms underlying vaccine effectiveness.
Keywords: complement; hybrid immunity; mpox virus; neutralizing antibodies; smallpox vaccination.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., Osborne J.C., Rampling T., Beadsworth M.B., Duncan C.J., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

