Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury
- PMID: 37196676
- PMCID: PMC10307625
- DOI: 10.1038/s41586-023-06191-5
Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury
Erratum in
-
Author Correction: Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury.Nature. 2025 Dec;648(8093):E11. doi: 10.1038/s41586-025-09955-3. Nature. 2025. PMID: 41310315 Free PMC article. No abstract available.
Abstract
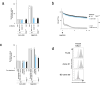
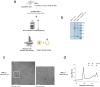
Plasma membrane rupture (PMR) in dying cells undergoing pyroptosis or apoptosis requires the cell-surface protein NINJ11. PMR releases pro-inflammatory cytoplasmic molecules, collectively called damage-associated molecular patterns (DAMPs), that activate immune cells. Therefore, inhibiting NINJ1 and PMR may limit the inflammation that is associated with excessive cell death. Here we describe an anti-NINJ1 monoclonal antibody that specifically targets mouse NINJ1 and blocks oligomerization of NINJ1, preventing PMR. Electron microscopy studies showed that this antibody prevents NINJ1 from forming oligomeric filaments. In mice, inhibition of NINJ1 or Ninj1 deficiency ameliorated hepatocellular PMR induced with TNF plus D-galactosamine, concanavalin A, Jo2 anti-Fas agonist antibody or ischaemia-reperfusion injury. Accordingly, serum levels of lactate dehydrogenase, the liver enzymes alanine aminotransaminase and aspartate aminotransferase, and the DAMPs interleukin 18 and HMGB1 were reduced. Moreover, in the liver ischaemia-reperfusion injury model, there was an attendant reduction in neutrophil infiltration. These data indicate that NINJ1 mediates PMR and inflammation in diseases driven by aberrant hepatocellular death.
© 2023. The Author(s).
Conflict of interest statement
The following authors are employees of Genentech: N.K., I.B.S., K.A., I.D., S.W., Z.L., O.S.K., B.L.L., J.Z., J.L., E.S., W.P.L., K.S., W.L., D.S., T.B., C.C., M.C.J., P.J., K.N., J.D.W., R.L.K. and V.M.D.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

