PRMT7 can prevent neurovascular uncoupling, blood-brain barrier permeability, and mitochondrial dysfunction in repetitive and mild traumatic brain injury
- PMID: 37196697
- PMCID: PMC10960645
- DOI: 10.1016/j.expneurol.2023.114445
PRMT7 can prevent neurovascular uncoupling, blood-brain barrier permeability, and mitochondrial dysfunction in repetitive and mild traumatic brain injury
Abstract
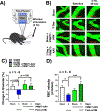
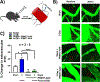
Mild traumatic brain injury (TBI) comprises the largest percentage of TBI-related injuries, with pathophysiological and functional deficits that persist in a subset of TBI patients. In our three-hit paradigm of repetitive and mild traumatic brain injury (rmTBI), we observed neurovascular uncoupling via decreased red blood cell velocity, microvessel diameter, and leukocyte rolling velocity 3 days post-rmTBI via intra-vital two-photon laser scanning microscopy. Furthermore, our data suggest increased blood-brain barrier (BBB) permeability (leakage), with corresponding decrease in junctional protein expression post-rmTBI. Mitochondrial oxygen consumption rates (measured via Seahorse XFe24) were also altered 3 days post-rmTBI, along with disrupted mitochondrial dynamics of fission and fusion. Overall, these pathophysiological findings correlated with decreased protein arginine methyltransferase 7 (PRMT7) protein levels and activity post-rmTBI. Here, we increased PRMT7 levels in vivo to assess the role of the neurovasculature and mitochondria post-rmTBI. In vivo overexpression of PRMT7 using a neuronal specific AAV vector led to restoration of neurovascular coupling, prevented BBB leakage, and promoted mitochondrial respiration, altogether to suggest a protective and functional role of PRMT7 in rmTBI.
Keywords: Bioenergetics; Blood-brain barrier; Cerebral blood flow; Gliosis; Leukocyte rolling; Mitochondrial dysfunction; Protein arginine methyltransferase; Traumatic brain injury.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
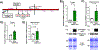


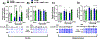



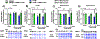
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

