Role of Procalcitonin as a Biomarker in Early Identification of Adverse Events Following Esophageal Atresia Surgery
- PMID: 37197237
- PMCID: PMC10185041
- DOI: 10.4103/jiaps.jiaps_156_21
Role of Procalcitonin as a Biomarker in Early Identification of Adverse Events Following Esophageal Atresia Surgery
Abstract
Introduction: Surgical complication following esophageal atresia repair is one of the several factors known to influence the final outcomes. Early identification of such complications may help in timely institution of therapeutic measures and translate into improved prognosis.
Objective: The objective of this study was to evaluate the role of procalcitonin in early prediction of the adverse events after surgery in patients of esophageal atresia and the temporal relationship with clinical manifestations and other inflammatory biomarkers such as C-reactive protein (CRP).
Materials and methods: This was a prospective study on consecutive patients of esophageal atresia (n = 23). Serum procalcitonin and CRP levels were assessed at baseline (prior to surgery) and on postoperative days (POD) 1, 3, 5, 7, and 14. The trends in the biomarker values and temporal relationships of deviation in trend with the clinical and conventional laboratory parameters and patient outcomes were analyzed.
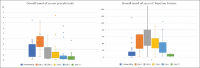
Results: Baseline serum procalcitonin was elevated (n = 23; 1.7 ng/ml: min: 0.07 ng/ml-max: 24.36 ng/ml) in 18/23 (78.3%) patients. Procalcitonin nearly doubled on POD-1 (n = 22; 3.28 ng/ml: min: 0.64 ng/ml-max: 16.51 ng/ml) followed by a gradual decline. CRP was also elevated on POD-1 (three times the baseline) and depicted a delayed peak at POD-3. POD-1 procalcitonin and CRP levels correlated with survival. POD-1 procalcitonin cutoff at 3.28 ng/ml predicted mortality with a sensitivity and specificity of 100% and 57.9% (P = 0.05). Serum procalcitonin and CRP were higher for patients who sustained complications, so was the time required for hemodynamic stabilization. Procalcitonin (baseline and POD-5) and CRP (POD-3 and POD-5) values correlated with the clinical course after surgery. Baseline procalcitonin cutoff at 2.91 ng/ml predicted the possibility of a major complication with a sensitivity of 71.4% and a specificity of 93.3%. POD-5 procalcitonin cutoff at 1.38 ng/ml predicted the possibility of a major complication with a sensitivity of 83.3% and a specificity of 93.3%. Patients who sustained major complications depicted a change in serum procalcitonin trend 24-48 h ahead of clinical manifestation of an adverse event.
Conclusions: Procalcitonin is a good indicator to identify the adverse events in neonates after surgery for esophageal atresia. The procalcitonin levels in patients who sustained a major complication depicted a reversal in trend 24-48 h of clinical manifestation. POD-1 procalcitonin correlated with survival while the baseline and POD-5 serum procalcitonin predicted the clinical course.
Keywords: Adverse event; C-reactive protein; biomarker; esophageal atresia; procalcitonin; sepsis; surgical complication.
Copyright: © 2023 Journal of Indian Association of Pediatric Surgeons.
Conflict of interest statement
There are no conflicts of interest.
Figures



Similar articles
-
Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program.Surg Endosc. 2018 Sep;32(9):4003-4010. doi: 10.1007/s00464-018-6144-x. Epub 2018 Mar 8. Surg Endosc. 2018. PMID: 29520440
-
Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients.Crit Care Med. 2000 Sep;28(9):3171-6. doi: 10.1097/00003246-200009000-00008. Crit Care Med. 2000. PMID: 11008977
-
Changes in serum procalcitonin, interleukin 6, interleukin 8 and C-reactive protein in neonates after surgery.Eur J Pediatr Surg. 2010 Jul;20(4):262-6. doi: 10.1055/s-0030-1253358. Epub 2010 May 3. Eur J Pediatr Surg. 2010. PMID: 20440673
-
The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills?Surg Infect (Larchmt). 2013 Dec;14(6):489-511. doi: 10.1089/sur.2012.028. Epub 2013 Nov 25. Surg Infect (Larchmt). 2013. PMID: 24274059 Review.
-
The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review.Crit Care. 2018 Nov 21;22(1):316. doi: 10.1186/s13054-018-2236-1. Crit Care. 2018. PMID: 30463590 Free PMC article.
References
-
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: Where do we stand. A review. Pediatr Neonatol. 2016;57:265–73. - PubMed
-
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. - PubMed
-
- Stocker M, Hop WC, van Rossum AM. Neonatal procalcitonin intervention study (NeoPInS): Effect of Procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: A multi-centre randomized superiority and non-inferiority Intervention Study. BMC Pediatr. 2010;10:89. - PMC - PubMed
-
- Stocker M, van Herk W, El Helou S, Dutta S, Fontana MS, Schuerman FA, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicentre, randomised controlled trial (NeoPIns) Lancet. 2017;390:871–81. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
