Modular One-Pot Strategy for the Synthesis of Heterobivalent Tracers
- PMID: 37197474
- PMCID: PMC10184157
- DOI: 10.1021/acsmedchemlett.3c00057
Modular One-Pot Strategy for the Synthesis of Heterobivalent Tracers
Abstract
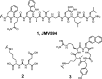
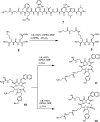
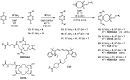
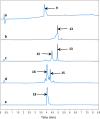
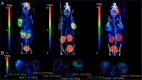
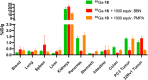
Bivalent ligands, i.e., molecules having two ligands covalently connected by a linker, have been gathering attention since the first description of their pharmacological potential in the early 80s. However, their synthesis, particularly of labeled heterobivalent ligands, can still be cumbersome and time-consuming. We herein report a straightforward procedure for the modular synthesis of labeled heterobivalent ligands (HBLs) using dual reactive 3,6-dichloro-1,2,4,5-tetrazine as a starting material and suitable partners for sequential SNAr and inverse electron-demand Diels-Alder (IEDDA) reactions. This assembly method conducted in a stepwise or in a sequential one-pot manner provides quick access to multiple HBLs. A conjugate combining ligands toward the prostate-specific membrane antigen (PSMA) and the gastrin-releasing peptide receptor (GRPR) was radiolabeled, and its biological activity was assessed in vitro and in vivo (receptor binding affinity, biodistribution, imaging) as an illustration that the assembly methodology preserves the tumor targeting properties of the ligands.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Portoghese P. S.; Larson D. L.; Sayre L. M.; Yim C. B.; Ronsisvalle G.; Tam S. W.; Takemori A. E. Opioid Agonist and Antagonist Bivalent Ligands. The Relationship between Spacer Length and Selectivity at Multiple Opioid Receptors. J. Med. Chem. 1986, 29 (10), 1855–1861. 10.1021/jm00160a010. - DOI - PubMed
-
- Huang G.; Pemp D.; Stadtmüller P.; Nimczick M.; Heilmann J.; Decker M. Design, Synthesis and in Vitro Evaluation of Novel Uni- and Bivalent Ligands for the Cannabinoid Receptor Type 1 with Variation of Spacer Length and Structure. Bioorg. Med. Chem. Lett. 2014, 24 (17), 4209–4214. 10.1016/j.bmcl.2014.07.038. - DOI - PubMed
-
- Lensing C. J.; Freeman K. T.; Schnell S. M.; Speth R. C.; Zarth A. T.; Haskell-Luevano C. Developing a Biased Unmatched Bivalent Ligand (BUmBL) Design Strategy to Target the GPCR Homodimer Allosteric Signaling (CAMP over β-Arrestin 2 Recruitment) Within the Melanocortin Receptors. J. Med. Chem. 2019, 62 (1), 144–158. 10.1021/acs.jmedchem.8b00238. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

