Immune checkpoint inhibitors beyond first-line progression with prior immunotherapy in patients with advanced non-small cell lung cancer
- PMID: 37197488
- PMCID: PMC10183556
- DOI: 10.21037/jtd-22-1611
Immune checkpoint inhibitors beyond first-line progression with prior immunotherapy in patients with advanced non-small cell lung cancer
Abstract
Background: Immunotherapy, monotherapy, and immunotherapy plus platinum-based chemotherapy are the standard treatments for advanced non-small cell lung cancer (NSCLC) patients with negative driver genes. However, the impact of similar continuing immunotherapy beyond progression (IBP) of first-line immunotherapy for advanced NSCLC has not yet been shown. This study aimed to estimate the impact of immunotherapy beyond first-line progression (IBF) and evaluate the factors associated with second-line efficacity.
Methods: Ninety-four cases of advanced NSCLC patients with progressive disease (PD) post first-line treatment with platinum-based chemotherapy plus immunotherapy and administrated prior immune checkpoint inhibitors (ICIs) between November 2017 and July 2021 were retrospectively analyzed. Survival curves were plotted using the Kaplan-Meier method. Cox proportional hazards regression analyses were applied to determine predictive factors independently associated with second-line efficacity.
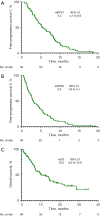
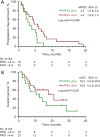
Results: A total of 94 patients were incorporated in this study. Patients who continued the original ICIs after initial PD were defined as IBF (n=42), whereas those who discontinued immunotherapy were defined as non-IBF (n=52). The second-line objective response rates (ORR, ORR = CR + PR) of patients in the IBF and non-IBF groups were 13.5% vs. 28.6%, respectively (P=0.070). No significant survival difference was found between patients in the IBF and non-IBF groups in first-line median progression-free survival (PFS) (mPFS1, 6.2 vs. 5.1 months, P=0.490), second-line median PFS (mPFS2, 4.5 vs. 2.6 months, P=0.216), or median overall survival (OS) (mOS, 14.4 vs. 8.3 months, P=0.188). However, the benefits inPFS2 were observed in individuals performed PFS1 >6 months (group A) than those of PFS1 ≤6 months (group B) (median PFS2, 4.6 vs. 3.2 months, P=0.038). Multivariate analyses did not reveal any independent prognostic factors for efficacy.
Conclusions: The benefits of continuing prior ICIs administration beyond first-line immunotherapy progression might not be obvious in patients with advanced NSCLC, but those first line treatment showed a longer period may receive efficacy benefits.
Keywords: Non-small cell lung cancer (NSCLC); efficacy; immune checkpoint inhibitors (ICIs); immunotherapy beyond progression (IBP).
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1611/coif). The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Efficacy and safety analysis of immune checkpoint inhibitor rechallenge therapy in locally advanced and advanced non-small cell lung cancer: a retrospective study.J Thorac Dis. 2024 Mar 29;16(3):1787-1803. doi: 10.21037/jtd-23-1767. Epub 2024 Mar 19. J Thorac Dis. 2024. PMID: 38617775 Free PMC article.
-
Efficacy and safety of maintenance immune checkpoint inhibitors with or without pemetrexed in advanced non-squamous non-small cell lung cancer: a retrospective study.BMC Cancer. 2022 May 24;22(1):576. doi: 10.1186/s12885-022-09674-2. BMC Cancer. 2022. PMID: 35606756 Free PMC article.
-
Treatment patterns and outcomes of immunotherapy in extensive-stage small-cell lung cancer based on real-world practice.Thorac Cancer. 2022 Dec;13(23):3295-3303. doi: 10.1111/1759-7714.14684. Epub 2022 Oct 11. Thorac Cancer. 2022. PMID: 36218023 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): a systematic literature review.Oncoimmunology. 2020 Jun 16;9(1):1774314. doi: 10.1080/2162402X.2020.1774314. Oncoimmunology. 2020. PMID: 32923134 Free PMC article.
-
Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis.Thorac Cancer. 2021 Apr;12(7):1058-1066. doi: 10.1111/1759-7714.13867. Epub 2021 Feb 14. Thorac Cancer. 2021. PMID: 33586297 Free PMC article.
Cited by
-
Dual-Modal Aptasensor for Sensitive Detection of Non-Small Cell Lung Cancer Exosomes Utilizing Two-Dimensional Nanopaper Co@g-C3N4@PB.ACS Omega. 2024 Aug 2;9(32):34493-34506. doi: 10.1021/acsomega.4c02346. eCollection 2024 Aug 13. ACS Omega. 2024. PMID: 39157104 Free PMC article.
-
Continuous immunotherapy beyond disease progression in patients with advanced non-small cell and small cell lung cancer.Cancer Immunol Immunother. 2025 Feb 25;74(4):124. doi: 10.1007/s00262-025-03958-9. Cancer Immunol Immunother. 2025. PMID: 39998635 Free PMC article.
-
Immunotherapy beyond progression following first‑line chemotherapy plus immunotherapy in advanced non‑small cell lung cancer: A retrospective study.Oncol Lett. 2024 Dec 5;29(2):90. doi: 10.3892/ol.2024.14836. eCollection 2025 Feb. Oncol Lett. 2024. PMID: 39677413 Free PMC article.
-
Continuation of same programmed death-1 inhibitor regime beyond progression is a novel option for advanced gastric cancer.BMC Cancer. 2024 Oct 18;24(1):1292. doi: 10.1186/s12885-024-13063-2. BMC Cancer. 2024. PMID: 39425079 Free PMC article.
References
-
- Chai Y, Wu X, Zou Y, et al. Immunotherapy combined with chemotherapy versus chemotherapy alone as the first-line treatment of PD-L1-negative and driver-gene-negative advanced nonsquamous non-small-cell lung cancer: An updated systematic review and meta-analysis. Thorac Cancer 2022;13:3124-32. 10.1111/1759-7714.14664 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
