Onasemnogene abeparvovec in spinal muscular atrophy: predictors of efficacy and safety in naïve patients with spinal muscular atrophy and following switch from other therapies
- PMID: 37197706
- PMCID: PMC10184045
- DOI: 10.1016/j.eclinm.2023.101997
Onasemnogene abeparvovec in spinal muscular atrophy: predictors of efficacy and safety in naïve patients with spinal muscular atrophy and following switch from other therapies
Abstract
Background: Efficacy and safety of onasemnogene abeparvovec (OA) for Spinal Muscular Atrophy infants under 7 months and <8.5 kg has been reported in clinical trials. This study examines efficacy and safety predictors in a wide age (22 days-72 months) and weight (3.2-17 kg) range, also including patients previously treated with other drugs.
Methods: 46 patients were treated for 12 months between January 2020 and March 2022. Safety profile was also available for another 21 patients with at least 6 month follow-up after OA infusion. 19/67 were treatment naïve when treated with OA. Motor function was measured with the CHOP-INTEND.
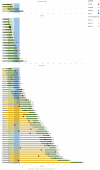
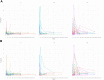
Findings: CHOP-INTEND changes varied among age groups. Baseline score and age at OA treatment best predicted changes. A mixed model post-hoc analysis showed that in patients treated before the age of 24 months the CHOP-INTEND changes were already significant 3 months after OA while in those treated after the age of 24 months the difference was only significant 12 months after OA. Adverse events occurred in 51/67. The risk for elevated transaminases serum levels was higher in older patients. This was also true for weight and for pre-treatment with nusinersen when analysed individually. A binomial negative regression analysis showed that only age at OA treatment had a significant effect on the risk of elevated transaminases.
Interpretation: Our paper describes OA 12-month follow-up showing efficacy across various age and weight groups not targeted by clinical trials. The study identifies prognostic factors for safety and efficacy in treatment selection.
Funding: None.
Keywords: Follow-up; Gene therapy; Longitudinal; Safety; Spinal muscular atrophy.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
No author has received financial support for the present manuscript. MP, GC, SM, RM, CP reported personal fees from Biogen, Novartis and Roche outside the submitted work; BB reported personal fees from Biogen and Novartis outside the submitted work; AdA reported personal fees from AveXis outside the submitted work; FR reported personal fees from Biogen outside the submitted work; DG, DL, CD, CT reported personal fees from Novartis outside the submitted work; MF reported personal fees from Sanofi and Amicus outside the submitted work; SC reported personal fees from Novartis and Scholar Rock outside the submitted work; RDS reported personal fees from Biogen and Roche outside the submitted work; RF reported personal fees from AveXis, Biogen, Capricor, families of SMA, Ionis Pharmaceuticals, Novartis, Roche, and ScholarRock outside the submitted work; EM reported personal fees from Biogen, Roche, Novartis, Scholar Rock, Epirium and Cytochinetics outside the submitted work; AC, AV, VAS, CA, CB, AP, RO, MR, IB, MS, CD, EA, CT, NB, EB have nothing to disclose.
Figures

References
-
- Mercuri E., Bertini E., Iannaccone S.T. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11(5):443–452. - PubMed
-
- Mercuri E., Sumner C.J., Muntoni F., Darras B.T., Finkel R.S. Spinal muscular atrophy. Nat Rev Dis Primers. 2022;8(1):52. - PubMed
-
- Mendell J.R., Al-Zaidy S., Shell R., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

