A novel insight on SARS-CoV-2 S-derived fragments in the control of the host immunity
- PMID: 37198208
- PMCID: PMC10191404
- DOI: 10.1038/s41598-023-29588-8
A novel insight on SARS-CoV-2 S-derived fragments in the control of the host immunity
Erratum in
-
Author Correction: A novel insight on SARS-CoV-2 S-derived fragments in the control of the host immunity.Sci Rep. 2023 Jul 25;13(1):12012. doi: 10.1038/s41598-023-39134-1. Sci Rep. 2023. PMID: 37491374 Free PMC article. No abstract available.
Abstract
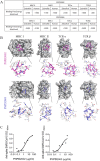
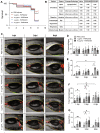
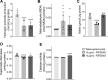
Despite all efforts to combat the pandemic of COVID-19, we are still living with high numbers of infected persons, an overburdened health care system, and the lack of an effective and definitive treatment. Understanding the pathophysiology of the disease is crucial for the development of new technologies and therapies for the best clinical management of patients. Since the manipulation of the whole virus requires a structure with an adequate level of biosafety, the development of alternative technologies, such as the synthesis of peptides from viral proteins, is a possible solution to circumvent this problem. In addition, the use and validation of animal models is of extreme importance to screen new drugs and to compress the organism's response to the disease. Peptides derived from recombinant S protein from SARS-CoV-2 were synthesized and validated by in silico, in vitro and in vivo methodologies. Macrophages and neutrophils were challenged with the peptides and the production of inflammatory mediators and activation profile were evaluated. These peptides were also inoculated into the swim bladder of transgenic zebrafish larvae at 6 days post fertilization (dpf) to mimic the inflammatory process triggered by the virus, which was evaluated by confocal microscopy. In addition, toxicity and oxidative stress assays were also developed. In silico and molecular dynamics assays revealed that the peptides bind to the ACE2 receptor stably and interact with receptors and adhesion molecules, such as MHC and TCR, from humans and zebrafish. Macrophages stimulated with one of the peptides showed increased production of NO, TNF-α and CXCL2. Inoculation of the peptides in zebrafish larvae triggered an inflammatory process marked by macrophage recruitment and increased mortality, as well as histopathological changes, similarly to what is observed in individuals with COVID-19. The use of peptides is a valuable alternative for the study of host immune response in the context of COVID-19. The use of zebrafish as an animal model also proved to be appropriate and effective in evaluating the inflammatory process, comparable to humans.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

