Targeting strategies for bone diseases: signaling pathways and clinical studies
- PMID: 37198232
- PMCID: PMC10192458
- DOI: 10.1038/s41392-023-01467-8
Targeting strategies for bone diseases: signaling pathways and clinical studies
Abstract
Since the proposal of Paul Ehrlich's magic bullet concept over 100 years ago, tremendous advances have occurred in targeted therapy. From the initial selective antibody, antitoxin to targeted drug delivery that emerged in the past decades, more precise therapeutic efficacy is realized in specific pathological sites of clinical diseases. As a highly pyknotic mineralized tissue with lessened blood flow, bone is characterized by a complex remodeling and homeostatic regulation mechanism, which makes drug therapy for skeletal diseases more challenging than other tissues. Bone-targeted therapy has been considered a promising therapeutic approach for handling such drawbacks. With the deepening understanding of bone biology, improvements in some established bone-targeted drugs and novel therapeutic targets for drugs and deliveries have emerged on the horizon. In this review, we provide a panoramic summary of recent advances in therapeutic strategies based on bone targeting. We highlight targeting strategies based on bone structure and remodeling biology. For bone-targeted therapeutic agents, in addition to improvements of the classic denosumab, romosozumab, and PTH1R ligands, potential regulation of the remodeling process targeting other key membrane expressions, cellular crosstalk, and gene expression, of all bone cells has been exploited. For bone-targeted drug delivery, different delivery strategies targeting bone matrix, bone marrow, and specific bone cells are summarized with a comparison between different targeting ligands. Ultimately, this review will summarize recent advances in the clinical translation of bone-targeted therapies and provide a perspective on the challenges for the application of bone-targeted therapy in the clinic and future trends in this area.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
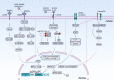

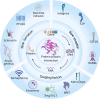
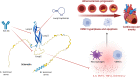

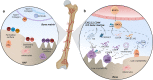

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

