Acute and Chronic Macrophage Differentiation Modulates TREM2 in a Personalized Alzheimer's Patient-Derived Assay
- PMID: 37198381
- PMCID: PMC10333375
- DOI: 10.1007/s10571-023-01351-7
Acute and Chronic Macrophage Differentiation Modulates TREM2 in a Personalized Alzheimer's Patient-Derived Assay
Abstract
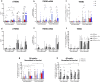
Neuroinflammation plays a pivotal role in the pathogenesis of Alzheimer`s disease (AD). Brain macrophage populations differentially modulate the immune response to AD pathology according to the disease stage. Triggering receptor expressed on myeloid cells 2 (TREM2) is known to play a protective role in AD and has been postulated as a putative therapeutic target. Whether, and to which extent TREM2 expression can be modulated in the aged macrophage population of the brain is unknown, emphasizing the need for a human, patient-specific model. Using cells from AD patients and matched controls (CO) we designed an assay based on monocyte-derived macrophages to mimic brain-infiltrating macrophages and to assess the individualized TREM2 synthesis in vitro. We systematically assessed the effects of short-term (acute-2 days) and long-term (chronic-10 days) M1- (LPS), M2- (IL-10, IL-4, TGF-β), and M0- (vehicle) macrophage differentiation on TREM2 synthesis. Moreover, the effects of retinoic acid (RA), a putative TREM2 modulator, on individualized TREM2 synthesis were assessed. We report increased TREM2 synthesis after acute M2- compared to M1-differentiation in CO- but not AD-derived cells. Chronic M2- and M0-differentiation however resulted in an increase of TREM2 synthesis in both AD- and CO-derived cells while chronic M1-differentiation increased TREM2 in AD-derived cells only. Moreover, chronic M2- and M0-differentiation improved the amyloid-β (Aβ) uptake of the CO-derived whereas M1-differentiation of the AD-derived cells. Interestingly, RA-treatment did not modulate TREM2. In the age of personalized medicine, our individualized model could be used to screen for potential drug-mediated treatment responses in vitro. Triggering receptor expressed on myeloid cells 2 (TREM2) has been postulated as a putative therapeutic target in Alzheimer's disease (AD). Using cells from AD patients and matched controls (CO), we designed a monocyte-derived macrophages (Mo-MФs) assay to assess the individualized TREM2 synthesis in vitro. We report increased TREM2 synthesis after acute M2- compared to M1- macrophage differentiation in CO- but not AD-derived cells. Chronic M2- and M0- differentiation however resulted in an increase of TREM2 synthesis in both AD- and CO-derived cells while chronic M1-differentiation increased TREM2 in AD-cells only.
Keywords: LOAD; Monocyte-derived macrophages; Patient-derived personalized assay; Retinoic acid; TREM2.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Byers DE, Wu K, Dang-Vu G, Jin X, Agapov E, Zhang X, Battaile JT, Schechtman K, Yusen R, Pierce RA, Holtzman MJ (2018) Triggering receptor expressed on myeloid cells-2 expression tracks With M2-like macrophage activity and disease severity in COPD. Chest 153(1):77–86. 10.1016/J.CHEST.2017.09.044 - PMC - PubMed
-
- Calvin CM, de Boer C, Raymont V, Gallacher J, Koychev I (2020) Prediction of Alzheimer’s disease biomarker status defined by the “ATN framework” among cognitively healthy individuals: results from the EPAD longitudinal cohort study. Alzheimer’s Res Therapy 12(1):143. 10.1186/S13195-020-00711-5/FIGURES/3 - PMC - PubMed
-
- Casati M, Ferri E, Gussago C, Mazzola P, Abbate C, Bellelli G, Mari D, Cesari M, Arosio B (2018) Increased expression of TREM2 in peripheral cells from mild cognitive impairment patients who progress into Alzheimer’s disease. Eur J Neurol 25(6):805–810. 10.1111/ENE.13583 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

