Single-cell and spatial transcriptomics: deciphering brain complexity in health and disease
- PMID: 37198436
- PMCID: PMC10191412
- DOI: 10.1038/s41582-023-00809-y
Single-cell and spatial transcriptomics: deciphering brain complexity in health and disease
Abstract
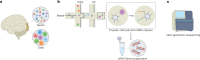
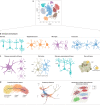
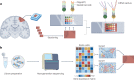
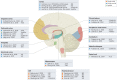
In the past decade, single-cell technologies have proliferated and improved from their technically challenging beginnings to become common laboratory methods capable of determining the expression of thousands of genes in thousands of cells simultaneously. The field has progressed by taking the CNS as a primary research subject - the cellular complexity and multiplicity of neuronal cell types provide fertile ground for the increasing power of single-cell methods. Current single-cell RNA sequencing methods can quantify gene expression with sufficient accuracy to finely resolve even subtle differences between cell types and states, thus providing a great tool for studying the molecular and cellular repertoire of the CNS and its disorders. However, single-cell RNA sequencing requires the dissociation of tissue samples, which means that the interrelationships between cells are lost. Spatial transcriptomic methods bypass tissue dissociation and retain this spatial information, thereby allowing gene expression to be assessed across thousands of cells within the context of tissue structural organization. Here, we discuss how single-cell and spatially resolved transcriptomics have been contributing to unravelling the pathomechanisms underlying brain disorders. We focus on three areas where we feel these new technologies have provided particularly useful insights: selective neuronal vulnerability, neuroimmune dysfunction and cell-type-specific treatment response. We also discuss the limitations and future directions of single-cell and spatial RNA sequencing technologies.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

