Establishing the safety of selective digestive decontamination within the ICU population: a bridge too far?
- PMID: 37198636
- PMCID: PMC10189709
- DOI: 10.1186/s13063-023-07356-3
Establishing the safety of selective digestive decontamination within the ICU population: a bridge too far?
Abstract
Background: Infection prevention interventions within the intensive care unit (ICU) setting, whether studied within quality improvement projects or cluster randomized trials (CRT), are seen as low risk and grounded in an ethical imperative. Selective digestive decontamination (SDD) appears highly effective at preventing ICU infections within randomized concurrent control trials (RCCTs) prompting mega-CRTs with mortality as the primary endpoint.
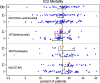
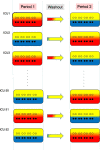
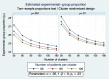
Findings: Surprisingly, the summary results of RCCTs versus CRTs differ strikingly, being respectively, a 15-percentage-point versus a zero-percentage-point ICU mortality difference between control versus SDD intervention groups. Multiple other discrepancies are equally puzzling and contrary to both prior expectations and the experience within population-based studies of infection prevention interventions using vaccines. Could spillover effects from SDD conflate the RCCT control group event rate differences and represent population harm? Evidence that SDD is fundamentally safe to concurrent non-recipients in ICU populations is absent. A postulated CRT to realize this, the SDD Herd Effects Estimation Trial (SHEET), would require > 100 ICUs to achieve sufficient statistical power to find a two-percentage-point mortality spillover effect. Moreover, as a potentially harmful population-based intervention, SHEET would pose novel and insurmountable ethical issues including who is the research subject; whether informed consent is required and from whom; whether there is equipoise; the benefit versus the risk; considerations of vulnerable groups; and who should be the gatekeeper?
Conclusion: The basis for the mortality difference between control and intervention groups of SDD studies remains unclear. Several paradoxical results are consistent with a spillover effect that would conflate the inference of benefit originating from RCCTs. Moreover, this spillover effect would constitute to herd peril.
Keywords: Antibiotic prophylaxis; Bacteremia; Herd peril; Intensive care; Mechanical ventilation; Selective digestive decontamination; Study design.
© 2023. The Author(s).
Conflict of interest statement
The author has no conflicts of interest to declare related to the material presented in this manuscript.
Figures
Similar articles
-
Studies of selective digestive decontamination as a natural experiment to evaluate topical antibiotic prophylaxis and cephalosporin use as population-level risk factors for enterococcal bacteraemia among ICU patients.J Antimicrob Chemother. 2019 Oct 1;74(10):3087-3094. doi: 10.1093/jac/dkz300. J Antimicrob Chemother. 2019. PMID: 31355880
-
Selective oropharyngeal decontamination versus selective digestive decontamination in critically ill patients: a meta-analysis of randomized controlled trials.Drug Des Devel Ther. 2015 Jul 14;9:3617-24. doi: 10.2147/DDDT.S84587. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26203227 Free PMC article. Review.
-
Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial.JAMA. 2014 Oct 8;312(14):1429-1437. doi: 10.1001/jama.2014.7247. JAMA. 2014. Retracted and republished in: JAMA. 2017 Apr 18;317(15):1583-1584. doi: 10.1001/jama.2017.1282. PMID: 25271544 Retracted and republished. Clinical Trial.
-
Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis.Clin Microbiol Infect. 2018 May;24(5):505-513. doi: 10.1016/j.cmi.2017.08.019. Epub 2017 Sep 1. Clin Microbiol Infect. 2018. PMID: 28870727
-
Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: a meta-analysis.BMC Infect Dis. 2014 Dec 31;14:714. doi: 10.1186/s12879-014-0714-x. BMC Infect Dis. 2014. PMID: 25551776 Free PMC article. Review.
Cited by
-
Does selective digestive decontamination (SDD) increase antibiotic resistance? Long-term comparison of two intensive care units (with and without SDD) of the same tertiary hospital.Eur J Clin Microbiol Infect Dis. 2024 May;43(5):885-893. doi: 10.1007/s10096-024-04792-0. Epub 2024 Mar 9. Eur J Clin Microbiol Infect Dis. 2024. PMID: 38460030 Free PMC article.
-
Indirect (herd) effects of topical antibiotic prophylaxis and oral care versus non-antimicrobial methods increase mortality among ICU patients: realigning Cochrane review data to emulate a three-tier cluster randomised trial.BMJ Open. 2023 Nov 30;13(11):e064256. doi: 10.1136/bmjopen-2022-064256. BMJ Open. 2023. PMID: 38035749 Free PMC article. Review.
-
Visualizing and diagnosing spillover within randomized concurrent controlled trials through the application of diagnostic test assessment methods.BMC Med Res Methodol. 2024 Aug 16;24(1):182. doi: 10.1186/s12874-024-02296-1. BMC Med Res Methodol. 2024. PMID: 39152400 Free PMC article.
-
Intestinal Microbiota Dysbiosis Role and Bacterial Translocation as a Factor for Septic Risk.Int J Mol Sci. 2025 Feb 26;26(5):2028. doi: 10.3390/ijms26052028. Int J Mol Sci. 2025. PMID: 40076650 Free PMC article. Review.
-
Trends in ICU mortality and underlying risk over three decades among mechanically ventilated patients. A group level analysis of cohorts from infection prevention studies.Ann Intensive Care. 2023 Jul 11;13(1):62. doi: 10.1186/s13613-023-01159-0. Ann Intensive Care. 2023. PMID: 37432605 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

