Dipeptidyl peptidase-1 inhibition with brensocatib reduces the activity of all major neutrophil serine proteases in patients with bronchiectasis: results from the WILLOW trial
- PMID: 37198686
- PMCID: PMC10189992
- DOI: 10.1186/s12931-023-02444-z
Dipeptidyl peptidase-1 inhibition with brensocatib reduces the activity of all major neutrophil serine proteases in patients with bronchiectasis: results from the WILLOW trial
Abstract
Background: Brensocatib is an oral, selective, reversible inhibitor of dipeptidyl peptidase-1 (DPP-1), responsible for activating neutrophil serine proteases (NSPs) including neutrophil elastase (NE), proteinase 3 (PR3), and cathepsin G (CatG). In chronic inflammatory lung diseases such as non-cystic fibrosis bronchiectasis (NCFBE), neutrophils accumulate in the airways resulting in excess active NSPs that cause damaging inflammation and lung destruction.
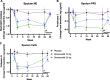
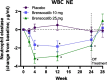
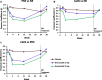
Methods: The 24-week WILLOW trial (NCT03218917) was a randomized, double-blind, placebo-controlled, parallel-group trial in patients with NCFBE conducted at 116 sites across 14 countries. In this trial, treatment with brensocatib was associated with improvements in clinical outcomes including time to first exacerbation, reduction in exacerbation frequency and a reduction in NE activity in sputum. An exploratory analysis of NE activity in white blood cell (WBC) extracts and NE, PR3 and CatG activity in sputum was conducted to further characterize brensocatib's effect and identify potential correlated effects.
Results: NE, PR3 and CatG activities were reduced in sputum and NE activity was reduced in WBC extracts in a dose-dependent manner after four weeks of brensocatib treatment, with a return to baseline four weeks after the end of treatment. Brensocatib produced the greatest reduction in the sputum activity of CatG, followed by NE and then PR3. Positive correlations among the sputum NSPs were observed both at baseline and in response to treatment, with the strongest correlation among the sputum NSPs for NE and CatG.
Conclusions: These results suggest a broad anti-inflammatory effect of brensocatib underlying its clinical efficacy observed in NCFBE patients.
Trial registration: The study was approved by the corresponding ethical review boards of all participating centers. The trial was approved by the Food and Drug Administration and registered at clinicaltrials.gov (NCT03218917) on July 17, 2017 and approved by the European Medicines Agency and registered at the European Union Clinical trials Register (EudraCT No. 2017-002533-32). An independent, external data and safety monitoring committee (comprising physicians with pulmonary expertise, a statistician experienced in the evaluation of clinical safety, and experts in periodontal disease and dermatology) reviewed all adverse events.
Keywords: Brensocatib; Cathepsin G; Dipeptidyl peptidase-1 inhibitor; Neutrophil elastase; Neutrophil serine protease; Non-cystic fibrosis bronchiectasis; Proteinase 3; Sputum biomarkers.
© 2023. The Author(s).
Conflict of interest statement
DC, JS, DL, CF, AT, KGM, JZ, WP and EJS are employees of Insmed Incorporated, Bridgewater, New Jersey. JDC has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Zambon, and Insmed Incorporated; a grant from Gilead; and personal fees from Novartis and Chiesi. BK has received personal fees from Insmed Incorporated.
Figures







References
-
- Cole PJ. Inflammation: a two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

