Malaria vaccines: the 60-year journey of hope and final success-lessons learned and future prospects
- PMID: 37198702
- PMCID: PMC10189698
- DOI: 10.1186/s41182-023-00516-w
Malaria vaccines: the 60-year journey of hope and final success-lessons learned and future prospects
Abstract
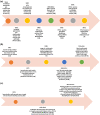
Background: The world has made great strides towards beating malaria, although about half of the world population is still exposed to the risk of contracting malaria. Developing an effective malaria vaccine was a huge challenge for medical science. In 2021 the World Health Organization (WHO) approved the first malaria vaccine, RTS,S/AS01 vaccine (Mosquirix™), for widespread use. This review highlights the history of development, and the different approaches and types of malaria vaccines, and the literature to date. It covers the developmental stages of RTS,S/AS01 and recommends steps for its deployment. The review explores other potential vaccine candidates and their status, and suggests options for their further development. It also recommends future roles for vaccines in eradicating malaria. Questions remain on how RTS,S vaccine will work in widespread use and how it can best be utilized to benefit vulnerable communities.
Conclusion: Malaria vaccines have been in development for almost 60 years. The RTS,S/AS01 vaccine has now been approved, but cannot be a stand-alone solution. Development should continue on promising candidates such as R21, PfSPZ and P. vivax vaccines. Multi-component vaccines may be a useful addition to other malaria control techniques in achieving eradication of malaria.
Keywords: Approval; Blood-stage vaccines; Challenges; Control; Development; Elimination; Erythrocytic vaccines; History; Implementation program; Malaria; Plasmodium falciparum; Pre-erythrocytic vaccines; RTS,S; Transmission blocking vaccines; Vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures



References
-
- WHO. World malaria report . 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 2020.
-
- WHO. Towards a global action plan for healthy lives and well-being for all. Geneva: World Health Organization. 2018.
-
- WHO. Malaria fact sheet 2021. Geneva: World Health Organization; 2021.
-
- WHO. WHO approves first malaria vaccine: why did it take so long? Geneva: World Health Organization. 2021.
Publication types
LinkOut - more resources
Full Text Sources
