Treatment patterns and cost estimations of systemic chemotherapy for pancreatic cancer in Japan: A retrospective database study
- PMID: 37199073
- PMCID: PMC10358248
- DOI: 10.1002/cam4.6100
Treatment patterns and cost estimations of systemic chemotherapy for pancreatic cancer in Japan: A retrospective database study
Abstract
Background: This study aimed to clarify the treatment patterns of pancreatic cancer patients receiving systemic chemotherapy in Japan and to estimate the direct medical costs in actual practice.
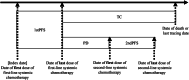
Research design and methods: This retrospective cohort study used electronic health record data between April 2008 and December 2018 in Japan. Participants had a confirmed pancreatic cancer diagnosis and received at least one systemic chemotherapy, including FOLFIRINOX, gemcitabine plus nab-paclitaxel, gemcitabine, and S-1. The outcomes were treatment patterns and monthly medical costs and the distribution of monthly medical costs across healthcare resource categories.
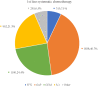
Results: Of the 4514 selected patients, 40.7%, 7.1%, 24.4%, and 21.3% used gemcitabine plus nab-paclitaxel, FOLFIRINOX, gemcitabine, and S-1 as first-line chemotherapy, respectively. The median monthly medical costs were the highest in the first month, with gemcitabine plus nab-paclitaxel ranking first (6813 USD), followed by FOLFIRINOX, gemcitabine, and S-1. The health resource categories with the highest shares of monthly medical costs during the first-line treatment period with gemcitabine plus nab-paclitaxel and FOLFIRINOX were hospitalization costs (FOLFIRINOX: 41%-37%; gemcitabine plus nab-paclitaxel: 40%-34%) and medicine costs (FOLFIRINOX: 51%-42%; gemcitabine plus nab-paclitaxel: 49%-38%).
Conclusions: This study sheds light on the current treatment patterns and direct medical costs of systemic chemotherapy for pancreatic cancer in Japan.
Keywords: Japan; cost estimation; pancreatic cancer; utilization.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Manabu Akazawa received honoraria and manuscript fees from Jansen, GSK, Takeda, and Shionogi. Other authors declare having no conflicts of interest directly relevant to the content of this article. Affiliations of Yuki Takumoto, Ryotaro Shibahara, and Hajime Asami are as of March 2023.
Figures
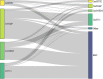
References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and moetality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941‐1953. - PubMed
-
- Foundation for Promotion of Cancer Research . Cancer Statistics in Japan‐2019 . 2019. Accessed August 5, 2022. https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statisti...
-
- Cancer Information Service, National Cancer Center, Japan . Annual Report of Hospital‐Based Cancer Registries . Accessed August 5, 2022. https://ganjoho.jp/public/qa_links/report/hosp_c/pdf/2020_report.pdf
-
- Japan Pancreas Society . Clinical Practice Guidelines for Pancreatic Cancer 2019 . 2019. Accessd August 5, 2022 [Japanese]. http://www.suizou.org/pdf/pancreatic_cancer_cpg‐2019.pdf
-
- Uesaka K, Boku N, Fukutom A, et al. JASPAC 01 study group. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer, a phase 3, open‐label, randomized, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248‐257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

