Impact of viral hepatitis therapy in multiple myeloma and other monoclonal gammopathies linked to hepatitis B or C viruses
- PMID: 37199121
- PMCID: PMC10772493
- DOI: 10.3324/haematol.2023.283096
Impact of viral hepatitis therapy in multiple myeloma and other monoclonal gammopathies linked to hepatitis B or C viruses
Abstract
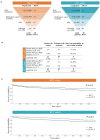
Subsets of multiple myeloma (MM) and monoclonal gammopathies of undetermined significance (MGUS) present with a monoclonal immunoglobulin specific for hepatitis C virus (HCV), thus are presumably HCV-driven, and antiviral treatment can lead to the disappearance of antigen stimulation and improved control of clonal plasma cells. Here we studied the role of hepatitis B virus (HBV) in the pathogenesis of MGUS and MM in 45 HBV-infected patients with monoclonal gammopathy. We analyzed the specificity of recognition of the monoclonal immunoglobulin of these patients and validated the efficacy of antiviral treatment (AVT). For 18 of 45 (40%) HBV-infected patients, the target of the monoclonal immunoglobulin was identified: the most frequent target was HBV (n=11), followed by other infectious pathogens (n=6) and glucosylsphingosine (n=1). Two patients whose monoclonal immunoglobulin targeted HBV (HBx and HBcAg), implying that their gammopathy was HBV-driven, received AVT and the gammopathy did not progress. AVT efficacy was then investigated in a large cohort of HBV-infected MM patients (n=1367) who received or did not receive anti-HBV treatments and compared to a cohort of HCV-infected MM patients (n=1220). AVT significantly improved patient probability of overall survival (P=0.016 for the HBV-positive cohort, P=0.005 for the HCV-positive cohort). Altogether, MGUS and MM disease can be HBV- or HCV-driven in infected patients, and the study demonstrates the importance of AVT in such patients.
Figures


Comment in
-
Exploring the role of viral hepatitis in plasma cell disorders.Haematologica. 2024 Jan 1;109(1):19-20. doi: 10.3324/haematol.2023.283461. Haematologica. 2024. PMID: 37470153 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

