Six-month immune responses to mRNA-1273 vaccine in combination antiretroviral therapy treated late presenter people with HIV according to previous SARS-CoV-2 infection
- PMID: 37199415
- PMCID: PMC10355808
- DOI: 10.1097/QAD.0000000000003585
Six-month immune responses to mRNA-1273 vaccine in combination antiretroviral therapy treated late presenter people with HIV according to previous SARS-CoV-2 infection
Abstract
Objective: Immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines in people with HIV (PWH) with a history of late presentation (LP) and their durability have not been fully characterized.
Design: In this prospective, longitudinal study, we sought to assess T-cell and humoral responses to SARS-CoV-2 mRNA vaccination up to 6 months in LP-PWH on effective combination antiretroviral therapy (cART) as compared to HIV-negative healthcare workers (HCWs), and to evaluate whether previous SARS-CoV-2 infection modulates immune responses to vaccine.
Methods: SARS-CoV-2 spike (S)-specific T-cell responses were determined by two complementary flow cytometry methodologies, namely activation-induced marker (AIM) assay and intracellular cytokine staining (ICS), whereas humoral responses were measured by ELISA [anti-receptor binding domain (RBD) antibodies) and receptor-binding inhibition assay (spike-ACE2 binding inhibition activity), before vaccination (T0), 1 month (T1) and 5 months (T2) after the second dose.
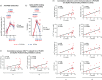
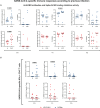
Results: LP-PWH showed at T1 and T2 significant increase of: S-specific memory and circulating T follicular helper (cTfh) CD4 + T cells; polyfunctional Th1-cytokine (IFN-γ, TNF-α, IL-2)- and Th2-cytokine (IL-4)-producing S-specific CD4 + T cells; anti-RBD antibodies and spike-ACE2 binding inhibition activity. Immune responses to vaccine in LP-PWH were not inferior to HCWs overall, yet S-specific CD8 + T cells and spike-ACE2 binding inhibition activity correlated negatively with markers of immune recovery on cART. Interestingly, natural SARS-CoV-2 infection, while able to sustain S-specific antibody response, seems less efficacious in inducing a T-cell memory and in boosting immune responses to vaccine, possibly reflecting an enduring partial immunodeficiency.
Conclusions: Altogether, these findings support the need for additional vaccine doses in PWH with a history of advanced immune depression and poor immune recovery on effective cART.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Comment in
-
Antibody and T-cell responses elicited by coronavirus disease 2019 vaccination in people with HIV-1: the case of late presenters.AIDS. 2023 Aug 1;37(10):1625-1627. doi: 10.1097/QAD.0000000000003624. AIDS. 2023. PMID: 37450628 Free PMC article. No abstract available.
References
-
- Inciarte A, Gonzalez-Cordon A, Rojas J, Torres B, de Lazzari E, de la Mora L, et al. . Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS 2020; 34:1775–1780. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous