Bone and Renal Health in Infants With or Without Breastmilk Exposure to Tenofovir-Based Maternal Antiretroviral Treatment in the PROMISE Randomized Trial
- PMID: 37199427
- PMCID: PMC10337310
- DOI: 10.1097/QAI.0000000000003218
Bone and Renal Health in Infants With or Without Breastmilk Exposure to Tenofovir-Based Maternal Antiretroviral Treatment in the PROMISE Randomized Trial
Abstract
Background: We assessed bone and kidney outcomes in infants randomized postdelivery as mother-infant pairs within the IMPAACT PROMISE trial to maternal tenofovir disoproxil fumarate-based antiretroviral treatment (mART) or infant nevirapine prophylaxis (iNVP) to prevent breastfeeding HIV transmission.
Methods: Infants were coenrolled in the P1084s substudy on randomization day and followed through Week 74. Lumbar spine bone mineral content (LS-BMC) was assessed at entry (6-21 age days) and Week 26 by dual-energy x-ray absorptiometry. Creatinine clearance (CrCl) was calculated at entry; Weeks 10, 26, and 74. Student t tests compared mean LS-BMC and CrCl at Week 26 and mean change from entry between arms.
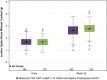
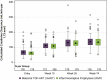
Results: Of 400 enrolled infants, the mean (SD; n) for entry LS-BMC was 1.68 g (0.35; n = 363) and CrCl was 64.2 mL/min/1.73 m 2 (24.6; n = 357). At Week 26, 98% of infants were breastfeeding and 96% on their assigned HIV prevention strategy. The mean (SD) Week 26 LS-BMC was 2.64 g (0.48) for mART and 2.77 g (0.44) for iNVP; mean difference (95% confidence interval [CI]) -0.13 g (-0.22 to -0.04), P = 0.007, n = 375/398 (94%). Mean absolute (-0.14 g [-0.23 to -0.06]) and percent (-10.88% [-18.53 to -3.23]) increase in LS-BMC from entry was smaller for mART than iNVP. At Week 26, the mean (SD) CrCl was 130.0 mL/min/1.73 m 2 (34.9) for mART vs. 126.1 mL/min/1.73 m 2 (30.0) for iNVP; mean difference (95% CI) 3.8 (-3.0 to 10.7), P = 0.27, n = 349/398 (88%).
Conclusion: Week 26 mean LS-BMC was lower in infants in the mART group compared with the iNVP group. However, this difference (∼0.23 g) was less than one-half SD, considered potentially clinically relevant. No infant renal safety concerns were observed.
Trial registration: ClinicalTrials.gov NCT01061151.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures
References
-
- Sattari M, Serwint JR, Levine DM. Maternal implications of breastfeeding: a review for the internist. Am J Med. 2019;132:912–920. - PubMed
-
- Schirm E, Schwagermann MP, Tobi H, et al. . Drug use during breastfeeding. A survey from The Netherlands. Eur J Clin Nutr. 2004;58:386–390. - PubMed
-
- World Health Organization. Consolidated Guidelines On Hiv Prevention, Testing, Treatment, Service Delivery And Monitoring: Recommendations For A Public Health Approach. World Health Organization; 2021. Available at: https://apps.who.int/iris/handle/10665/351172. Accessed March 22, 2022. - PubMed
-
- The British HIV Association. BHIVA Guidelines for the Treatment of HIV-1-Positive Adults with Antiretroviral Therapy 2015; 2016. Available at: https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-inter.... Accessed March 22, 2022.
-
- EACS European AIDS Clinical Society. EACS Guidelines 11.0. Brussels, Belgium: EACS European AIDS Clinical Society; 2021.

