Vesicular Stomatitis Virus (VSV) G Glycoprotein Can Be Modified to Create a Her2/Neu-Targeted VSV That Eliminates Large Implanted Mammary Tumors
- PMID: 37199666
- PMCID: PMC10308914
- DOI: 10.1128/jvi.00372-23
Vesicular Stomatitis Virus (VSV) G Glycoprotein Can Be Modified to Create a Her2/Neu-Targeted VSV That Eliminates Large Implanted Mammary Tumors
Abstract
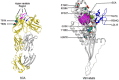
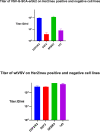
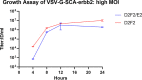
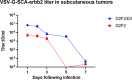
Viral oncolytic immunotherapy is a nascent field that is developing tools to direct the immune system to find and eliminate cancer cells. Safety is improved by using cancer-targeted viruses that infect or grow poorly on normal cells. The recent discovery of the low-density lipoprotein (LDL) receptor as the major vesicular stomatitis virus (VSV) binding site allowed for the creation of a Her2/neu-targeted replicating recombinant VSV (rrVSV-G) by eliminating the LDL receptor binding site in the VSV-G glycoprotein (gp) and adding a sequence coding for a single chain antibody (SCA) to the Her2/neu receptor. The virus was adapted by serial passage on Her2/neu-expressing cancer cells resulting in a virus that yielded a 15- to 25-fold higher titer following in vitro infection of Her2/neu+-expressing cell lines than that of Her2/neu-negative cells (~1 × 108/mL versus 4 × 106 to 8 × 106/mL). An essential mutation resulting in a higher titer virus was a threonine-to-arginine change that produced an N-glycosylation site in the SCA. Infection of Her2/neu+ subcutaneous tumors yielded >10-fold more virus on days 1 and 2 than Her2/neu- tumors, and virus production continued for 5 days in Her2/neu+ tumors compared with 3 days that of 3 days in Her2/neu- tumors. rrVSV-G cured 70% of large 5-day peritoneal tumors compared with a 10% cure by a previously targeted rrVSV with a modified Sindbis gp. rrVSV-G also cured 33% of very large 7-day tumors. rrVSV-G is a new targeted oncolytic virus that has potent antitumor capabilities and allows for heterologous combination with other targeted oncolytic viruses. IMPORTANCE A new form of vesicular stomatitis virus (VSV) was created that specifically targets and destroys cancer cells that express the Her2/neu receptor. This receptor is commonly found in human breast cancer and is associated with a poor prognosis. In laboratory tests using mouse models, the virus was highly effective at eliminating implanted tumors and creating a strong immune response against cancer. VSV has many advantages as a cancer treatment, including high levels of safety and efficacy and the ability to be combined with other oncolytic viruses to enhance treatment results or to create an effective cancer vaccine. This new virus can also be easily modified to target other cancer cell surface molecules and to add immune-modifying genes. Overall, this new VSV is a promising candidate for further development as an immune-based cancer therapy.
Keywords: targeted vesicular stomatitis virus; viral oncoimmunotherapy; viral oncolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu.Int J Cancer. 2007 Jul 15;121(2):425-30. doi: 10.1002/ijc.22680. Int J Cancer. 2007. PMID: 17354238
-
Rapid adaptation of a recombinant vesicular stomatitis virus to a targeted cell line.J Virol. 2006 Sep;80(17):8603-12. doi: 10.1128/JVI.00142-06. J Virol. 2006. PMID: 16912309 Free PMC article.
-
Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors.Cancer Gene Ther. 2009 Jan;16(1):44-52. doi: 10.1038/cgt.2008.55. Epub 2008 Jul 25. Cancer Gene Ther. 2009. PMID: 18654610
-
Oncolytic Therapy of Solid Tumors by Modified Vesicular Stomatitis Virus.DNA Cell Biol. 2024 Feb;43(2):57-60. doi: 10.1089/dna.2023.0368. Epub 2023 Dec 11. DNA Cell Biol. 2024. PMID: 38079267 Review.
-
Phase I study of VSV-GP (BI 1831169) as monotherapy or combined with ezabenlimab in advanced and refractory solid tumors.Future Oncol. 2022 Aug;18(24):2627-2638. doi: 10.2217/fon-2022-0439. Epub 2022 Jun 14. Future Oncol. 2022. PMID: 35699077 Review.
Cited by
-
A nanobody interaction with SARS-COV-2 Spike allows the versatile targeting of lentivirus vectors.J Virol. 2024 Sep 17;98(9):e0079524. doi: 10.1128/jvi.00795-24. Epub 2024 Aug 29. J Virol. 2024. PMID: 39207135 Free PMC article.
-
Distinguishing Protein and Gene Delivery Enables Characterization and Bioengineering of Extracellular Vesicle-Adeno-Associated Virus Vectors.bioRxiv [Preprint]. 2025 Jul 4:2025.07.02.662894. doi: 10.1101/2025.07.02.662894. bioRxiv. 2025. PMID: 40631339 Free PMC article. Preprint.
-
CARG-2020 targets IL-12, IL-17, and PD-L1 pathways to effectively treat melanoma and breast cancer.Sci Rep. 2025 Aug 13;15(1):29649. doi: 10.1038/s41598-025-14750-1. Sci Rep. 2025. PMID: 40804279 Free PMC article.
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

