Environmental and Taxonomic Drivers of Bacterial Extracellular Vesicle Production in Marine Ecosystems
- PMID: 37199672
- PMCID: PMC10304870
- DOI: 10.1128/aem.00594-23
Environmental and Taxonomic Drivers of Bacterial Extracellular Vesicle Production in Marine Ecosystems
Abstract
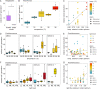
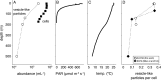
Extracellular vesicles are small (approximately 50 to 250 nm in diameter), membrane-bound structures that are released by cells into their surrounding environment. Heterogeneous populations of vesicles are abundant in the global oceans, and they likely play a number of ecological roles in these microbially dominated ecosystems. Here, we examine how vesicle production and size vary among different strains of cultivated marine microbes as well as explore the degree to which this is influenced by key environmental variables. We show that both vesicle production rates and vesicle sizes significantly differ among cultures of marine Proteobacteria, Cyanobacteria, and Bacteroidetes. Further, these properties vary within individual strains as a function of differences in environmental conditions, such as nutrients, temperature, and light irradiance. Thus, both community composition and the local abiotic environment are expected to modulate the production and standing stock of vesicles in the oceans. Examining samples from the oligotrophic North Pacific Gyre, we show depth-dependent changes in the abundance of vesicle-like particles in the upper water column in a manner that is broadly consistent with culture observations: the highest vesicle abundances are found near the surface, where the light irradiances and the temperatures are the greatest, and they then decrease with depth. This work represents the beginnings of a quantitative framework for describing extracellular vesicle dynamics in the oceans, which is essential as we begin to incorporate vesicles into our ecological and biogeochemical understanding of marine ecosystems. IMPORTANCE Bacteria release extracellular vesicles that contain a wide variety of cellular compounds, including lipids, proteins, nucleic acids, and small molecules, into their surrounding environment. These structures are found in diverse microbial habitats, including the oceans, where their distributions vary throughout the water column and likely affect their functional impacts within microbial ecosystems. Using a quantitative analysis of marine microbial cultures, we show that bacterial vesicle production in the oceans is shaped by a combination of biotic and abiotic factors. Different marine taxa release vesicles at rates that vary across an order of magnitude, and vesicle production changes dynamically as a function of environmental conditions. These findings represent a step forward in our understanding of bacterial extracellular vesicle production dynamics and provide a basis for the quantitative exploration of the factors that shape vesicle dynamics in natural ecosystems.
Keywords: Alcanivorax; Alteromonas; Dokdonia; Marinobacter; North Pacific Gyre; Pelagibacter; Polaribacter; Prochlorococcus; Thalassospira; extracellular vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Biller SJ, Lundeen RA, Hmelo LR, Becker KW, Arellano AA, Dooley K, Heal KR, Carlson LT, Van Mooy BAS, Ingalls AE, Chisholm SW. 2022. Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbes. Environ Microbiol 24:420–435. doi:10.1111/1462-2920.15834. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

