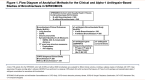
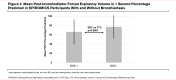
Impact of Bronchiectasis on COPD Severity and Alpha-1 Antitrypsin Deficiency as a Risk Factor in Individuals with a Heavy Smoking History
- PMID: 37199731
- PMCID: PMC10484491
- DOI: 10.15326/jcopdf.2023.0388
Impact of Bronchiectasis on COPD Severity and Alpha-1 Antitrypsin Deficiency as a Risk Factor in Individuals with a Heavy Smoking History
Abstract
Rationale: Bronchiectasis is common among those with heavy smoking histories, but risk factors for bronchiectasis, including alpha-1 antitrypsin deficiency, and its implications for COPD severity are uncharacterized in such individuals.
Objectives: To characterize the impact of bronchiectasis on COPD and explore alpha-1antitrypsin as a risk factor for bronchiectasis.
Methods: SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) participants (N=914; ages 40-80 years; ≥20-pack-year smoking) had high-resolution computed tomography (CT) scans interpreted visually for bronchiectasis, based on airway dilation without fibrosis or cicatrization. We performed regression-based models of bronchiectasis with clinical outcomes and quantitative CT measures. We deeply sequenced the gene encoding -alpha-1 antitrypsin, SERPINA1, in 835 participants to test for rare variants, focusing on the PiZ genotype (Glu366Lys, rs28929474).
Measurements and main results: We identified bronchiectasis in 365 (40%) participants, more frequently in women (45% versus 36%, p=0.0045), older participants (mean age=66[standard deviation (SD)=8.3] versus 64[SD=9.1] years, p=0.0083), and those with lower lung function (forced expiratory volume in 1 second [FEV1 ] percentage predicted=66%[SD=27] versus 77%[SD=25], p<0.0001; FEV1 to forced vital capacity [FVC] ratio=0.54[0.17] versus 0.63[SD=0.16], p<0.0001). Participants with bronchiectasis had greater emphysema (%voxels ≤-950 Hounsfield units, 11%[SD=12] versus 6.3%[SD=9], p<0.0001) and parametric response mapping functional small airways disease (26[SD=15] versus 19[SD=15], p<0.0001). Bronchiectasis was more frequent in the combined PiZZ and PiMZ genotype groups compared to those without PiZ, PiS, or other rare pathogenic variants (N=21 of 40 [52%] versus N=283 of 707[40%], odds ratio [OR]=1.97; 95% confidence interval [CI]=1.002, 3.90, p=0.049), an association attributed to White individuals (OR=1.98; 95%CI = 0.9956, 3.9; p=0.051).
Conclusions: Bronchiectasis was common in those with heavy smoking histories and was associated with detrimental clinical and radiographic outcomes. Our findings support alpha-1antitrypsin guideline recommendations to screen for alpha-1 antitrypsin deficiency in an appropriate bronchiectasis subgroup with a significant smoking history.
Keywords: COPD; alpha-1 antitrypsin; bronchiectasis; lung function.
JCOPDF © 2023.
Conflict of interest statement
Drs. Izquierdo, Marion, Genese, O’Neal, Li, Hawkins, Kanner, Tejwani, Zein, and Meyers have nothing to disclose. Dr. Newell reports grants from the National Institutes of Health (NIH), serving as a medical advisor with a consulting income, patents, and stock options with VIDA, and book royalties from Elsevier. Dr. Barjaktarevic reports personal fees from AstraZeneca, Boehringer Ingelheim, Grifols, Verona Pharma, and GSK; grants from AMGEN and grants and personal fees from GE Healthcare and Mylan/Theravance, outside the submitted work. Dr. Barr reports grants from the NIH, the Foundation for the NIH, and the COPD Foundation, during the conduct of the study and grants from the Alpha-1 Foundation, and personal fees from UpToDate, outside the submitted work. Dr. Cooper reports grants from the NIH/NHLBI and the Foundation for the NIH, during the conduct of the study, and personal fees from PulmonX, NUVAIRA, and MGC Diagnostics, and other from GSK outside the submitted work. Dr. Couper reports grants from the NHLBI and the COPD Foundation, during the conduct of the study. Dr. Curtis reports consulting fees paid to his institution from AstraZeneca PLC, Novartis AG, and CSL Behring LLC, outside this work; and personal travel support from AstraZeneca PLC, outside this work. Dr. Han reports personal fees from GSK, BI, AZ, Merck, and Mylan, and non-financial support from Novartis and Sunovion, outside the submitted work. Dr. Hansel reports grants and personal fees from AstraZeneca and GSK, grants from Boehringer Ingelheim, the NIH, and the COPD Foundation, and personal fees from Mylan, outside the submitted work. Dr. Martinez reports grants from the Department of Defense, during the conduct of the study; personal fees, non-financial support and other from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Inova Fairfax Health System, Miller Communications, the National Association for Continuing Education, Novartis, ProterrixBio, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rican Respiratory Society, Chiesi, Sunovion, Theravance, Potomac, University of Alabama-Birmingham, Physicians Education Resource, the Canadian Respiratory Network, Teva, and Dartmouth University; personal fees from Columbia University, MD Magazine, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape, the American Thoracic Society, Rockpointe, Rare Disease Healthcare Communications, the France Foundation, and Physicians Education Resource; other from Afferent/Merck,Biogen, Veracyte, Prometic, Bayer, Bridge Biotherapeutics, ProMedior, and Gala; non-financial support from Gilead, Nitto, and Zambon; personal fees and other from Patara/Respivant and grants from the NIH outside the submitted work. Dr. Paine reports grants from the NHLBI, and the COPD Foundation, during the conduct of the study; and grants from the Department of Veterans Affairs, outside the submitted work. Dr. Woodruff is a consultant for AstraZeneca, Theravance, Glenmark Pharmaceuticals, Sanofi, and Regeneron and has received funding from Genetech and the COPD Foundation. Dr. Hoffman is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. Dr. Peters reports grants from the NIH and the NHLBI during the conduct of the study; personal fees from Array Biopharma, Integrity CE, AstraZeneca, Aerocrine, Boehringer-Ingelheim, Experts in Asthma, Gilead, GSK, Merck, Novartis, Ono Pharmaceuticals, Pfizer, PPD Development, Quintiles, Sunovion, Saatchi and Saatichi, Targacept, TEVA, Theron, AstraZeneca, Sanofi, and Regeneron; and grants from the NIH and the NHLBI, outside the submitted work. Dr. Bleeker reports other from an NIH grant, clinical trials through his employers, Wake Forest School of Medicine and the University of Arizona for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson and Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme; personal fees serving as a paid consultant for AztraZeneca, MedImmune, Boehringer Ingelheim, GSK, Novartis, Regeneron, and Sanofi Genzyme outside the submitted work. Dr. Ortega reports grants from the NIH, personal fees from CSL Behring, and personal fees from Regeneron and Sanofi for its Independent Data and Monitoring Committee participation outside the submitted work.
References
-
- Ni Y,Shi G,Yu Y,Hao J,Chen T,Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465-1475. doi: https://doi.org/10.2147/COPD.S83910 - PMC - PubMed
-
- Hurst JR,Elborn JS,De Soyza A. BRONCH-UKConsortium. COPD-bronchiectasis overlap syndrome. Eur Respir J. 2015;45(2):310-313. doi: https://doi.org/10.1183/09031936.00170014 - PubMed
-
- Martinez-Garcia MA,Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401-1411. doi: https://doi.org/10.2147/COPD.S132961 - PMC - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2018 Report. GOLD website. Published 2018. Accessed January 2023. https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev...
-
- Stewart JI. Clinical impact of CT radiological feature of bronchiectasis in the COPDGene cohort. Am J Respir Crit Care Med. 2012;185:A3656. https://doi.org/10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A3656
Grants and funding
- HHSN268201100037C/HL/NHLBI NIH HHS/United States
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- R01 HL111527/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- K08 HL133381/HL/NHLBI NIH HHS/United States
- R01 HL142992/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous