Results from Expanded Access Programs: A Review of Academic Literature
- PMID: 37199856
- PMCID: PMC10193319
- DOI: 10.1007/s40265-023-01879-4
Results from Expanded Access Programs: A Review of Academic Literature
Abstract
Background: Although expanded access is an increasingly used pathway for patients to access investigational medicine, little is known on the magnitude and content of published scientific research collected via expanded access.
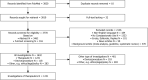
Methods: We performed a review of all peer-reviewed expanded access publications between January 1, 2000 and January 1, 2022. We analyzed the publications for drugs, diseases, disease area, patient numbers, time, geographical location, subject, and research methodology (single center/multicenter, international/national, prospective/retrospective). We additionally analyzed endpoints reported in all COVID-19-related expanded access publications.
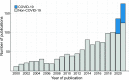
Results: We screened 3810 articles and included 1231, describing 523 drugs for 354 diseases for 507,481 patients. The number of publications significantly increased over time ([Formula: see text]). Large geographical disparities existed as Europe and the Americas accounted for 87.4% of all publications, whereas Africa only accounted for 0.6%. Oncology and hematology accounted for 53% of all publications. Twenty-nine percent of all expanded access patients (N = 197,187) reported on in 2020 and 2021 were treated in the context of COVID-19.
Conclusions: By summarizing characteristics of patients, diseases, and research methods described in all scientific literature published on expanded access, we provide a unique dataset for future research. We show that published scientific research on expanded access has surged over the past decades, partly due to COVID-19. However, international collaboration and equity in geographic access remain an issue of concern. Lastly, we stress the need for harmonization of research legislation and guidance on the value of expanded access data within real-world data frameworks to improve equity in patient access and streamline future expanded access research.
© 2023. The Author(s).
Conflict of interest statement
CAU-dG has received unrestricted grants from Boehringer Ingelheim, Astellas, Celgene, Sanofi, Janssen-Cilag, Bayer, Amgen, Genzyme, Merck, Glycostem Therapeutics, Astra Zeneca, Roche, and Merck. TBP works part-time for expanded access service provider myTomorrows, in which he holds stock and stock options (< 0.01%). He is contractually free to publish, and the service provider is not involved in any of his past or ongoing research, nor in this manuscript. DGJC received payments for lectures for Takeda, and conference visit support from Servier, all outside the submitted work. JS, SSA, NK, and JVR do not report any conflicts of interest.
Figures


References
-
- Polak TB, van Rosmalen J, Uyl-De Groot CA. Response to open peer commentary "making it count: extracting real world data from compassionate use and expanded access programs". Am J Bioeth. 2020;20(11):W4–W5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

